The BioPhorum Operation Group’s (BPOG’s) Container Closure Integrity Testing (CCIT) workstream would like to congratulate the United States Pharmacopeia’s committee for its latest revision to USP chapter <1207> Package Integrity Evaluation: Sterile Products. Generally, we believe it provides a comprehensive overview of the available methods for container–closure testing and outlines many important elements for consideration in establishing a successful CCIT strategy. We first responded to the USP <1207> draft when it was released for comment in 2014. And from our…
Manufacturing
Elastomer Stoppers: Working Toward Adopting an Industry-Wide User Requirements Specification for Particulate Levels
Two years ago, the companies involved in the BioPhorum Operations Group (BPOG) fill–finish community agreed that the quality of elastomer stoppers for vials was causing problems for biopharmaceutical manufacturers. So they deemed it to be a priority for the group. The problem is particularly pronounced for vial stoppers used in legacy products, which may have been on the market for several years. Many such medicines remain valuable for large patient populations. The stoppers used on legacy medicines are manufactured using…
The First Single-Use Diaphragm Valve: Automated and Controllable Systems Increase Process Reliability
Single-use components and systems now are firmly established in the pharmaceutical and biotechnology industries. The trend toward simplified and flexible upstream and downstream plant design means that these components are becoming increasingly important — especially in biopharmaceutical production. In the past, the only available disposables were primarily tubes, fittings, and possibly filters. But the number of single-use systems has been increasing for a number of years now. It is hardly surprising that plant designers and operators now can rely on…
Design and Performance of Single-Use, Stirred-Tank Bioreactors
Single-use components and systems have been incorporated into many bioprocesses as an alternative to cleanable, reusable systems. A wide range of publications have detailed the reasons for this trend toward a single-use approach. Justification in many cases comes from process-specific benefits such as increased manufacturing flexibility — especially for contract manufacturing organizations (CMOs) — enhanced sterility assurance, elimination of cleaning, reduced capital investment, faster processing times with increased productivity, faster start-up, and other benefits (1). One critical factor in the…
Membrane-Based Clarification of Polysaccharide Vaccines
Polysaccharide vaccines are essential for protection against infectious diseases, which remain an alarming cause of mortality. The first glycoconjugate vaccine for use in humans — a Haemophilus influenzae type b (Hib) conjugate — was licensed in the United States in 1987. This vaccine successfully reduced the incidence of invasive Hib disease in childhood and led to the further development of conjugate vaccines designed to prevent infection by other encapsulated bacteria (1). Polysaccharides are relatively complex carbohydrates made up of many…
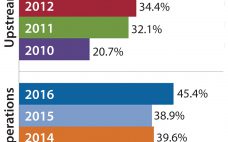
Outsourcing of Buffer Preparation Activity Is Increasing
The major fluid products used in bioprocessing — culture media and buffers — are classically prepared in-house by rehydrating (dissolving and mixing) powders purchased from suppliers. Most bioprocessing facilities consider in-house preparation of these fluids to be a core bioprocessing task. However, some companies are outsourcing the work either by purchasing preprepared materials from vendors or hiring contract manufacturing organizations (CMOs) to prepare them. Buffer fluid preparation is one area of downstream production operations that are seeing an increase in…
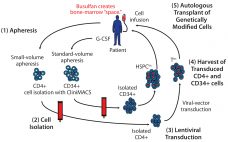
Cell-Delivered Gene Therapy: This Viral Vector Manufacturing Method Could Widen Its Applicability
Cell-delivered gene therapy is making an impact on a range of diseases (1–17). To date, successful treatments have generally been in conditions involving genetic deficiencies/abnormalities, for which introduction of a normal gene allele has been corrective (1–12, 18). Such an approach requires a vector containing the normal allele to overcome the mutant or lacking gene. The vector of choice for cell-delivered gene therapy is often a lentivirus that integrates and expresses introduced therapeutic genes in host target cells and their…
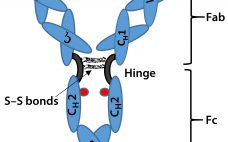
Biosimilar Therapeutic Monoclonal Antibodies: Gaps in Science Limit Development of an Industry Standard for Their Regulatory Approval, Part 2
Last month, Part 1 of this discussion briefly described the regulatory landscape for developing biosimilar therapeutic monoclonal antibodies (TMAbs). We identified certain specific structural components of TMAb drug substances that warrant particular attention because alterations to them are likely to affect therapeutic safety and effectiveness. Now we conclude by considering whether studies of reference materials can further the development of analytical industry standards to ensure comparability of putative biosimilar TMAbs with innovator TMAbs. We suggest that the time is right…
Special Report on Antibody-Drug Conjugates: Technical Challenges and Opportunities
Among the emerging targeted therapies in biotechnology, antibody–drug conjugates (ADCs) hold a unique position. An ADC consists of a monoclonal antibody (MAb) with affinity to tumor cells, a cytotoxic small-molecule payload, and a linker connecting the two. Together the MAb, conjugation chemistry, and cytotoxin increase the complexity of ADCs several-fold relative to unmodified MAbs — and exponentially relative to chemotherapies. Viewing ADCs as hybrids of antibody- and chemotherapy-based cancer therapies is tempting. That description applies chemically and structurally, but ADCs’…
Engineering Tissues with Bioprinting
Commonly referred to as three-dimensional (3D) printing, additive manufacturing encompasses a set of technologies that fabricate objects in an additive way, layer by layer, rather than conventional means of fabrications that generally subtract unwanted material from a larger block. Precise control over material placement allows 3D printing to fabricate objects that otherwise would not be manufacturable. Although many of these technologies have been around for two or three decades, recently they have received a significant amount of attention from industry,…