Induced pluripotent stem cells (iPSCs) are promising starting materials for several clinical and industrial applications in cell therapy production, drug discovery, and in vitro screening. But as I learned from Maxime Feyeux (cofounder and chief science officer of TreeFrog Therapeutics in Bordeaux, France), such cells are a relatively new option in the toolbox of cell therapy developers. Advanced-therapy products can require controlled proliferation and differentiation of millions or even billions of iPSCs depending on the clinical indication, he pointed out,…
Manufacturing
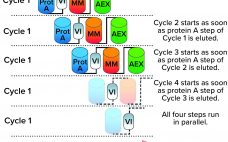
Reducing Downstream Scale-Up Needs: Advances Toward Continuous Downstream Processing
The biopharmaceutical industry generally acknowledges that upstream and downstream aspects of drug-substance manufacturing are experiencing a capacity mismatch. Today, many recombinant proteins can be produced at expression titers of 3 g/L, with some yields exceeding 10 g/L. Such titers represent 100-fold increases in production capability compared with values from twenty years ago (1, 2). Increases in cell-culture density and improvements to perfusion-mode bioreactor systems hold promise for increasing yields further still. Such developments, combined with the broad availability of concentrated…
Scaling AAV Production: Easing the Transition from Laboratory Scales to Commercial Manufacturing
Adenoassociated virus (AAV) has emerged as the leading vector for gene therapy delivery. Compared with options such as lentivirus and adenovirus, AAV exhibits a strong safety profile because it has low pathogenicity and requires a helper virus to replicate. AAV is also capable of long-term gene expression, and it can infect both dividing and nondividing cells (1–5). Developers of advanced therapies have found such advantages to be quite attractive. As of January 2021, two gene therapy products have gained US…
Smart, Real-Time Quality Insights Boost Life Sciences Manufacturing
The COVID-19 pandemic has shone a light on restrictive business processes, information silos, and poor supply-chain visibility in many sectors. In biopharmaceutical manufacturing, for example, difficulties associated with product-quality management have been exposed and starkly felt. However, public healthcare measures over the past 18 months have put physical distance between team members, thereby hampering the usual form-filling, manual sign-offs and spreadsheet-based recordkeeping associated with monitoring traditional manufacturing processes. In some cases, a lack of formal face-to-face discussions in the workplace…
A Missing Link to Achieving Higher Vaccination Rates in Developing Countries
Puerto Rico is leading COVID-19 vaccination efforts in the United States, with 89.7% of adults already fully vaccinated (1). However, many other regions are struggling to gain that level of traction, if any. Less than 1% of people in developing countries are fully vaccinated (2). Because Pfizer–BioNTech’s and Moderna’s respective mRNA-based SARS-CoV-2 vaccines still require ultracold temperatures for long-term storage, vaccine distribution in remote locations is arduous without appropriate cold-chain infrastructure. Puerto Rico’s success in vaccinating its population demonstrates how…
eBook: Gene Therapies —
Developers Slowly Emerge from a Pandemic
This eBook gauges shifting expectations for the gene therapy industry amid the COVID-related uncertainties and clinical setbacks of the past couple years. BioProcess Insider founding editor Dan Stanton reports on the January 2022 Phacilitate Advanced Therapies Week event, specifically a standing presentation on the 10 most important industry drivers from the past year. Since 2017, advancements in gene therapies have featured prominently in these presentations. In 2021, gene therapies again made the list, but this time for more troubling reasons,…
Increasing the Integrity of Closed Systems: Advantages Offered by Single-Use Connectors
Due to the complexity of biologic development and manufacturing and the business pressures of the biopharmaceutical industry’s landscape, the margin for error in today’s industry is small. That is why minimizing the threat of contamination is critical when using a closed system for drug development and manufacturing. Yet the traditional method of connecting each step in a closed process can present other risks to the integrity of your product. Therefore, it is important for you to be confident in selecting…
Bench to Bedside: Why Successful Drug Development Requires a Team Approach
The process from drug development to market approval takes many years and requires both integration and orchestration of several activities based on deep scientific and commercial expertise. We tend to think of drug development as a sequential, phase-driven process during which a product is taken methodically from discovery through preclinical studies, clinical development, and ultimately commercial launch. In reality, development of new drugs is not linear; rather, it requires integrated coordination between collaborators from different functions and stakeholders — from…
Antibody-Derivative Biotherapeutics: Fragments and Fusions Define the Future
Monoclonal antibodies (MAbs) remain the dominant biopharmaceutical product class, but as biotechnologies have advanced in recent decades, developers have found ways to exploit their “magic-bullet” capabilities while putting aside their limitations. That has led to a new generation of antibody-related therapeutics created by cutting and pasting molecular domains. Researchers are mixing and matching functional moieties of antibodies and other molecules to create custom-designed proteins with powerful efficacy and tunable targeting. A simple search at Taylor & Francis Online (https://www.tandfonline.com), the…
Development and Manufacture of Therapeutic Bispecific Antibodies
To meet the ongoing need for new and improved drugs, the biopharmaceutical community strives to create molecules with new functions. Bispecific antibodies (bsAbs), which can simultaneously home in on two different targets, illustrate the scientific ingenuity needed for this task. The basic proof of concept for these complex molecules was established in 1960 (1), and their application to the redirection of effector cells was reported in the mid-1980s (2–4), but producing them has proved to be challenging. Many technical advances,…