Greetings from the BPI editorial staff — we wish you a healthy and prosperous 2019! Beginning with a combined January–February issue somehow makes the year seem to be speeding by right from the start. We are immersed now in the first radical redesign of our magazine since the publication’s founding in 2003. Some aspects proceed smoothly; others, not so much. You see with this issue a new cover design that ties us in with our KNect365 event brands. You’ll see…
January-February 2019
January-February BioProcess Insider
Launched in June 2018, the BioProcess Insider digital information portal delivers the latest financial and business news and expert insider views influencing the commercialization of biopharmaceuticals. Here are just a few recent stories edited for our space limitations in print. For more discussion and in-depth analysis, check out the website at www.bioprocessinsider.com and sign up for the twice-weekly newsletter. AstraZeneca Closing Two Colorado Sites Anglo-Swedish pharmaceutical giant AstraZeneca is ending operations at its facilities in Boulder and Longmont, CO, less…
Monetizing Innovations in the Biopharmaceutical Industry
The biopharmaceutical industry has seen strong growth over the past 15 years, with a compounded annual growth rate (CAGR) of 6.7%. However, if you look at that growth in five-year intervals, it becomes apparent that the rates have begun to decline in many countries. Companies have withdrawn somewhat and put initiatives into place to curb further increases in the cost of drugs. The CAGR fell from 8.7% for the period of 2002–2006 to only 3.1% for 2012–2016. In our yearly…
Worldwide Biopharmaceutical CMO Capacity Analysis
Contract manufacturing organizations (CMOs) are a core part of the biopharmaceutical industry, with commercial manufacturing making up about a third of marketed products. Here we examine worldwide CMO biopharmaceutical manufacturing (bioprocessing) capacity, concentrating on “mainstream” CMOs — defined as those providing primarily mammalian cell culture or microbial fermentation services for manufacture at any scale of proteins and antibodies. This analysis follows our recent article examining worldwide bioprocessing capacity status and trends (1). Survey Methodology The 2018 15th Annual Report and…
Partnerships in Immunotherapy for the Future of Cancer Treatment
Immunotherapy seeks to harness the power of our human immune system to fight disease. In this rapidly evolving field, collaboration among different stakeholders is essential to bringing new treatments to market. Patient advocacy groups, researchers, hospitals, manufacturers, and government entities all are working together to translate promising new research into life-saving products. Types of immunotherapy include monoclonal antibodies (MAbs) and antibody derivatives, checkpoint inhibitors (immune-modulating proteins), cancer vaccines, T-cell therapies, and cytokines — so the approach involves a range of…
Development of Large-Scale Bulk Freezing Systems
The biopharmaceutical industry is under pressure to generate value for shareholders and find innovative therapies while driving down development and manufacturing costs. As the industry considers more flexible and scalable production solutions for patients, new therapies will continue to be developed faster than ever before. These products will need to rely on robust and reliable manufacturing solutions. To deliver on that challenge, streamlining manufacturing processes will be necessary so that many lots of drug substances can be manufactured, stored, and/or…
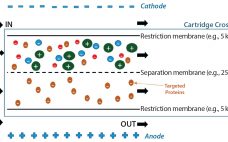
Tangential-Flow Electrophoresis: Investigation of Factors Involved in an Effective Separation of Human Serum Albumin from Human Plasma
Human plasma is a complex mixture of biomolecules such as serum albumin, immunoglobulins, coagulation factors, and others (1). These important protein biomolecules often are present at low levels or lacking in affected patients with certain life-threatening conditions. To extract those valuable proteins, a number of purification methods have been developed over time. Plasma fractionation can be traced back to the middle of the 20th century, when Edwin Cohn of Harvard University developed the first industrial process to purify proteins from…
A Brief History of Adherent Cell Culture: Where We Come From and Where We Should Go
In the past 20 years, novel therapeutics have become a major segment of biopharmaceutical research and development, particularly for immune disorders and cancer. Progress in gene therapies could bring cures for once deadly and debilitating genetic disorders such as hemophilia or muscular dystrophy. Biologic drug products offer potential treatments that have not been possible with traditional (chemistry-based) approaches. But such products also are more difficult to produce cost effectively at an industrial scale because of the intricacies associated with biological…
Qualitative and Quantitative Host Cell Protein Analysis Using Mass Spectrometry
Host cell proteins (HCPs) originate from host organisms that are used to produce biopharmaceutical products. They are in-process contaminants that must be minimized during downstream process operations. According to regulatory agencies, the maximum permitted level of total HCP in a biopharmaceutical product is 100 ng per mg (100 ppm) (1). HCPs can decrease drug efficacy and pose a risk to patient safety because they can bring on undesirable immune responses. Thus, HCPs are a critical quality attribute that should be…
Using Slope Spectroscopy Methods: Risk Assessment and Cost Savings
The biopharmaceutical industry’s need for rapid, accurate concentration measurements of protein-containing products is critical. The protein-concentration assay measures ultraviolet absorption at 280 nm (A280) and usually is performed both as an in-process test and for product-release testing. The SoloVPE system can analyze samples across a wide range of target concentrations without the need for labor-intensive and error-prone dilutions. Slope Spectroscopy methods provide companies with a universal platform for determining protein concentration for all in-process, clinical, and commercial methods. During in-process UV…