Performing viral clearance studies is an important safety element of manufacturing all biopharmaceuticals expressed from mammalian cells (1). Typically, viral clearance is described as a log reduction value (LRV) and calculated as the log10 of the ratio of input to output virus load. Amounts of virus load are calculated from the volume and concentration of input and output fractions. Virus concentration is often calculated as 50% of tissue-culture infective dose (TCID50) using the Spearman–Kärber (SK) equation (2, 3). In this…
Viral Clearance
A Response Plan for Viral Contamination in Bioproduction Facilities
The biopharmaceutical industry uses living biological systems as a platform for manufacturing of protein-based drugs, vaccines, and other therapies derived from or consisting of different cell types. On one hand, living systems are inherently susceptible to viral infection and may harbor endogenous viruses, so the potential for such contamination cannot be eliminated. On the other hand, the industry has an excellent patient-safety record. Viral safety is achieved through three fundamental measures: prevention (e.g., by selection), removal (by clearance and/or reduction),…
Detection and Clearance of Viruses in the Biopharmaceutical Industry
Viral contamination is a common threat to all animal- and human-derived biopharmaceuticals. This type of contamination can affect any part of a bioproduction process, so biomanufacturers need to perform viral testing studies and incorporate viral clearance methods into their processes. Viral contaminants can come from cell lines (e.g., endogenous retroviruses) or from adventitious (e.g., mycoplasma) introduction during drug manufacturing. Virus testing of master cell banks (MCBs), working cell banks (WCBs), end-of-production cell banks, and bulk unprocessed harvest material is called…
Monoclonal Antibody Aggregate Polish and Viral Clearance Using Hydrophobic-Interaction Chromatography
Hydrophobic Interaction chromatography (HIC) is a powerful polishing tool for the downstream purification and manufacture of biotherapeutics. HIC offers orthogonal selectivity for the clearance of difficult process and product-related impurities such as aggregates, host cell proteins and endogenous and adventitious viruses. In this study, a family of POROS HIC resins with novel ethyl and benzyl chemistries was used to successfully polish two clinical stage monoclonal antibodies harboring very high levels of product aggregation (>10%). In addition to aggregate removal, viral…
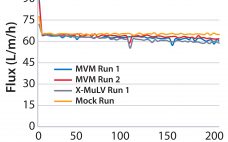
Modeling Virus Clearance: Use of a Noninfectious Surrogate of Mouse Minute Virus As a Tool for Evaluating an Anion-Exchange Chromatography Method
Viral safety is a critical focus during biopharmaceutical manufacturing (1–5). Although well-characterized mammalian cells such as the Chinese hamster ovary (CHO) line have been used for decades, both endogenous expression of retroviral-like particles and exogenous contamination events from viruses warrant continued vigilance (6, 7). International regulatory agencies require biomanufacturers to validate the “viral clearance” efficacy of their downstream manufacturing process steps before resulting products can be awarded clinical trial or commercial approval (8–10). Currently, viral clearance testing is based on…
The Complete e-Book of Biosafety Testing
Expect the expected. But plan for the unexpected. If your Biosafety Development takes a nose dive, Eurofins Lancaster Laboratories’ team of regulatory experts and experienced scientists will help you land safely on two feet. Download The Complete e-Book of Biosafety Testing to learn more about our expertise in biologics raw materials, cell bank preparation, adventitious virus testing, viral clearance studies, next-generation sequencing, genetic stability testing, and more. This e-Book contains the following chapters: Mitigating Risk and Reducing Regulatory Scrutiny of…
Inactivation of Enveloped Viruses: Seeking Alternatives to a Problematic Surfactant
Triton X-100 detergent makes an interesting case study in bioprocess sustainability strategy. Also known as octylphenol ethoxylate (OPE), this nonionic surfactant has many uses in biopharmaceutical research and development. Among other laboratory applications, it is used to lyse cells and DNA in research, to solubilize membrane proteins and decellularize animal-derived tissues, to reduce the surface tension of aqueous solutions during immunostaining, and to remove sodium dodecyl sulfate (SDS) from polyacrylamide gel electrophoresis (PAGE) gels for analysis. It also serves as…
Viral Risk Mitigation: A Global Regulatory Perspective
The production of biologics will always have the risk of viral contamination. Manufacturers have developed a multitiered approach — tailored to individual processes — to prevent adventitious viruses from entering production processes, detect contamination in raw materials and process intermediates, and remove viruses in downstream purification. This article provides an overview of the global regulatory framework to ensure the viral safety of biologics. Past Contamination Events Past contamination events have resulted in corrective and preventative actions to reduce the risk…
Virus Segregation During Purification Processes: Calculation of Critical Potential Carryover of Viruses
Before a pharmaceutical product is introduced into humans, either in a clinical trial or as a marketed product, virus safety must be evaluated carefully. Virus safety normally is ensured using a three step complementary approach: selecting and testing cell lines and/or raw materials for the absence of viruses, testing the product at appropriate steps of production, and assessing the capacity of a production process to clear infectious viruses (1). The latter (also referred to as viral clearance) is the subject herein. Spiking studies are conducted to evaluate the capacity of a purification…
Advanced Viral Clearance Study Design: A Total Viral Challenge Approach to Virus Filtration
Biologics derived from mammalian organisms have been accepted for therapeutic use for almost a century (1). However, these pharmaceuticals have the potential for contamination with pathogenic adventitious agents such as viruses. With cell-line–derived recombinant proteins, the viral risks commonly include viruses in the Retroviridae and Parvoviridae families (2). As patient safety and manufacturing facility suitability became significant concerns in the 1980s and 1990s, several industry and regulatory bodies reached consensus on how to approach the unique challenges of viral safety…