No longer are scientists bound to the time-consuming, error-prone use of dilution factors and fixed-pathlength measurements in determining the concentration of an analyte in solution. Using the slope spectroscopy technique, the Solo VPE system (from C Technologies) offers a new method of determining analyte concentration based on the Beer–Lambert law and slope derived from absorbance measurements made at multiple pathlengths (1). Mathematics: The Beer–Lambert law is expressed as A = αlc, where A is the measured absorbance, α is the…
Analytical
Expansion of Human Mesenchymal Stem Cells: Using Microcarriers and Human Platelet Lysate
Cell therapy holds the promise of delivering the next generation of future medical breakthroughs. In this respect, multipotent progenitor cells such as human mesenchymal stem cells (hMSCs) have attracted high clinical interest because of their ability to differentiate into various cell types and their immunoregulatory properties. Furthermore, hMSCs express only low levels of class I major histocompatibility complex (MHC I) molecules on their surfaces and are therefore invisible to a host’s immune system. Finally, hMSCs can actively suppress the innate…
A Novel Solid-Media E. coli Platform: Comparison with Standard Fermentation Processes
MicroProtein Technologies Inc. has developed the MPTxpress high-yield, low‑cost, recombinant Escherichia coli manufacturing platform. Rather than using liquid culture media within stirred bioreactors, the system uses trays filled with semisolid (gelled) culture media overlaid with or without a permeable membrane on which the E. coli is cultured. Compared with conventional liquid fermentation platforms, the MPTxpress system reduces the number of steps in up- and downstream processing and required infrastructure, significantly improves yields, and lowers costs. It provides simplicity for mixing…
Due Diligence of Early Stage Technologies: Achieving Rapid Product Development with Low R&D Costs
Increased understanding of human diseases at molecular and cellular levels is leading to development of novel life-science technologies. Such advancements typically pertain to discovery and manufacturing of novel human therapeutics, new modes of drug delivery, and novel diagnostic technologies. The majority of those technologies are developed by early stage biopharmaceutical companies that have a greater appetite for risk than do larger companies. Early stage biopharmaceutical companies, however, have limited capital raised through personal sources, angel investors, venture capital, or government…
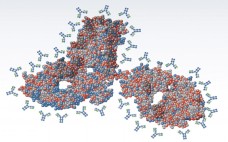
Higher-Order Structure Comparability: Case Studies of Biosimilar Monoclonal Antibodies
Great successes for monoclonal antibody (MAb)–based biologics over the past decade have provided many valuable options for patients combating some of the most serious diseases in the world, including cancer and autoimmune diseases. MAbs and antibody–drug conjugates (ADCs) are among the fastest growing biologic segments in development, with hundreds of candidates currently under clinical study. Meanwhile, society is facing the challenge of increasingly higher costs in healthcare including the cost of pharmaceuticals. With an aging population in many parts of…
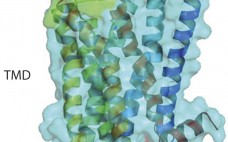
Targeting G Protein–Coupled Receptors with Biologics for Therapeutic Use, Part 1
G -protein coupled receptors (GPCRs) represent a target superfamily linked to many disorders across all therapeutic areas. Although this target class has been historically treated by small molecules and peptides, antibodies can offer a number of advantages over such molecules by virtue of their specificity, dosing frequency, and restricted penetration. They also can provide other functional effects specifically mediated by the Fc region (ADCC and CDC) as well as different modalities such as those offered by bispecific and antibody drug…
Site-Specific Characterization of Glycosylation on Protein Drugs
A large proportion of biotherapeutic products are glycoproteins. These include erythropoietin and other cytokines, antibodies, glycosyltransferases, and glycosidases, which together generate billions of dollars in sales worldwide. Such drugs are inherently complex. As new treatments emerge and biosimilars are evaluated, the need to better understand their molecular structures is more acute than ever. Therapeutic glycoproteins are typically produced as recombinant products in cell culture systems. Glycosylation is of major importance during development of these drugs because their glycan chains markedly…
Process Challenges of Antibody–Drug Conjugates
With two products now on the market, and a host of others in clinical trials, antibody-drug conjugates (ADCs) are slowly becoming a big business. Designed to deliver extremely active cytotoxic drugs that are otherwise undosable, they take advantage of the targeting ability of a specifically designed monoclonal antibody (MAb) to “shield” a highly potent API (HPAPI) as it travels through a patient’s bloodstream after administration. Once the antibody reaches its target on the cancer cell, it will release the payload,…
Qualification of Scale-Down Bioreactors: Validation of Process Changes in Commercial Production of Animal-Cell-Derived Products, Part 2 — Application
Here we apply our approach to validation of animal cell culture process changes using qualified, scale-down bioreactors. As described in Part 1 (including Table 1, Figures 1 and 2, and References 1–23), the goal is to facilitate implementation for the benefit of both the patients and industry. “Qualification of Scale-Down Bioreactors: Validation of Process Changes in Commercial Production of Animal-Cell–Derived Products, Part 1 — Concept” appears on pages 38–45 of BioProcess International’s May 2014 issue. Process changes often entail validation,…
Enabling Greater Process Control and Higher Protein Titers: Advances in Downstream Single-Use Technologies
Downstream protein purification (the stage in which a protein is isolated and purified) is one of the last steps in biotherapeutic manufacturing. Single-use technologies are an increasingly popular choice for both upstream and downstream bioprocessing because they offer significant benefits over traditional multiuse manufacturing systems. Single-use technologies also provide an array of logistical benefits, including reduced costs, minimized risk of cross-contamination, and improved operational efficiency (1). Challenges remain, however, in designing a complete, streamlined, single-use process for downstream protein purification.…