Benefits of single-use technologies over traditional stainless-steel solutions in biopharmaceutical manufacturing include reductions in set-up times, cleaning/cleaning validation costs, elimination of cross-contamination risks, and smaller operating footprints. But despite increasing adoption of such systems, concerns remain about extractable and leachable (E&L) compounds from plastic single-use systems (SUS) components with the potential to compromise the efficacy and safety of final drug products. Such concerns are magnified by the growing number of SUS suppliers and the complex supply chain for SUS and…
Analytical
Enhanced Galactosylation of Monoclonal Antibodies: Using Medium Supplements and Precursors of UDP-Galactose, Part 1
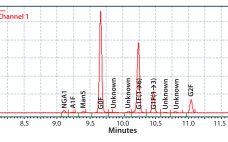
The biopharmaceutical industry needs better understanding of how monoclonal antibody (MAb) glycosylation is influenced by components in cultivation media — and it needs methods to exert some control over the structure of MAb glycans. That structure can affect MAb function. Thus, a high-throughput (HTP) assay is needed for characterizing MAb glycosylation so that developers can observe the effects of cultivation conditions on MAb glycosylation rapidly, with a goal of producing MAbs that have a desired glycan structure. The method also…
Therapeutic IgG-Like Bispecific Antibodies: Modular Versatility and Manufacturing Challenges, Part 2
Monoclonal antibodies (MAbs) are bivalent and monospecific, with two antigen-binding arms that both recognize the same epitope. Bispecific and multispecific antibodies, collectively referred to herein as bispecific antibodies (bsAbs), can have two or more antigen-binding sites, which are capable of recognizing and binding two or more unique epitopes. Based on their structure, bsAbs can be divided into two broad subgroups: IgG-like bsAbs and non–IgG-like bsAbs. We have chosen to focus on the former in this review. Part one provides a…
eBook: Addressing Quality in Cell-Line Development — Direct Analysis of Bioreactor Harvest for Clone Selection and Process Optimization
Using Direct Analysis of Bioreactor Harvest for Clone Selection and Process Optimization Therapeutic monoclonal antibodies (MAbs) mostly are manufactured using bioengineered mammalian cells cultured in a bioreactor for two to three weeks. High temperatures and an altered redox environment may compromise the quality of MAbs produced (e.g., fragmentation, truncation), as can the presence of proteases, reductases, and other chemicals released from dead cells. Thus, it would be valuable to establish analytical methods that can help cell culture groups monitor immunoglobulin…
An Approach to Generating Better Biosimilars: Considerations in Controlling Glycosylation Variability in Protein Therapeutics
The global market for biotherapeutics has expanded extensively over the past decade and is projected to account for more than a quarter of the pharmaceutical market by 2020, with sales exceeding US$290 billion (1). Continued expansion of the biosimilar marketplace has led to many commercial opportunities and technical challenges. The biological systems used to manufacture such drug products are inherently variable — a feature that has important consequences for the reproducibility, safety, and efficacy of the resulting products. Therefore, a…
eBook: Of Microbrews and Medicines — Understanding Their Similarities and Differences in Bioprocessing Can Help Improve Yields and Quality While Reducing Cost
Meeting a biopharmaceutical scientist or engineer who proclaims a love for brewing is not surprising. Perhaps it’s because of the challenge of mixing raw ingredients together and waiting patiently for the final product, maybe it’s the hands-on nature of the equipment or the data analytics entertainment, or it just might be the simple joy of creating something. Whatever attracts a scientist or engineer to making medicines and/or craft brews, a surprising number of principles hold true for both bioprocesses despite…
eBook: Bioinks for Bioprinting — Three-Dimensional Printing in Research and Medicine
Three-dimensional (3D) printing is one method of digital biomanufacturing for both basic biological research and translational, clinical applications. The medical field has used it to create such constructions as 3D surgical models for preoperative planning, to assist surgeons in their procedure preparations, which improves postsurgical outcomes. Examples here include generation of cleft-palate models (1), orthopedic applications (2), and cardiovascular surgical planning (3). Other forms of 3D printing for biological applications — such as 3D bioprinting — go beyond such surgical…
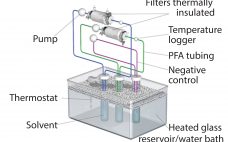
Implementation of the BPOG Extractables Testing Protocols: Working with Multiple Single-Use Components
Single-use technologies offer significant advantages over traditional stainless-steel solutions for biopharmaceutical manufacturing. Reductions in setup times, cleaning and cleaning-validation costs, elimination of cross-contamination risks, and smaller footprints are just some of the benefits they provide. Although adoption of single-use systems (SUS) for commercial manufacturing is expanding, concerns persist that extractable and leachable (E&L) compounds from plastic SUS components potentially can leach into final drug products and compromise efficacy and safety. Those concerns are magnified amid the growing number of SUS…
Simplify Upstream Process Development and Scale-Up: Single-Use 5:1 Turndown-Ratio Bioreactor Technology
Single-use technologies (SUTs) have been adopted widely in the biopharmaceutical industry for product development as well as clinical- and commercial-scale manufacturing. Over the years, suppliers of such equipment have addressed concerns about waste management, extractables and leachables, and reliability of supply — and as a result, end users have gained confidence in SUTs. Recognizing potential benefits that can be realized for both clinical and commercial operations, biomanufacturers increasingly are implementing SU solutions at larger scales in both upstream production and…
Integrated PAT Automated Feedback Control of Critical Process Parameters Using Modern In Situ Analytics
Simply put, the best way to control a critical process parameter (CPP) is to measure that specific parameter, integrate the live signal into your control system, and apply a smart feedback algorithm for an automated control loop. The challenge in doing this for bioprocesses has been due, in part, to the complex, highly dynamic, and variable nature of the process along with the lack of robust, scalable, and multiformat (single-use or multiuse) technologies that can monitor (in real time) such…