Computer-aided biology describes a growing ecosystem of tools that augment human capabilities in the laboratory. In this report we give two case study examples of how computer-aided biology has transformed industrial gene therapy bioprocessing. Read on to discover how Synthace’s Antha cloud-based software platform has enabled industrial collaborators Oxford Biomedica and the Cell and Gene Therapy Catapult to harness the power of Bioprocessing 4.0 by: incorporating new process analytical technologies (PAT), such as Raman Spectroscopy, into their unit operations automating…
2020
eBook: Development of Bioassays — A Report from the BEBPA’s First Virtual Meeting
Bioassay development is one of the most challenging aspects of biotherapeutic development. These tests are vital to providing an accurate picture of potency, stability, and biological activity. But bioassay development can be complex and expensive — and often test results can vary. Cell-based bioassays especially lack robustness. In March 2020, the Biopharmaceutical Emerging Best Practices Association (BEBPA) presented its annual bioassay conference in virtual format. Here, BPI’s senior technical editor reports on the conference’s discussions. Read on to learn more…
Patient Access Tops the List of Advanced Therapy Milestones at Phacilitate 2020
In a highly anticipated presentation at the 2020 Phacilitate Leaders World event — part of Advanced Therapies Week, along with the World Stem Cell Summit in Miami, FL — Susan Nichols (chief executive officer for Falcon Therapeutics), highlighted 10 events from 2019 that drove conversation, investment, and innovation in regenerative medicine. Although clustered regularly interspaced short palindromic repeats (CRISPR), business consolidations, and production capacity powered the cell and gene therapy (CGT) space in 2019, a new proactive focus on patient…
Discussions at Phacilitate 2020 on Business, Manufacturing, and Future Trends
Presenters in the three main program tracks at the Phacilitate Leaders World conference in Miami, FL, this past January represented sponsor-developers of cell/gene-therapy (CGT) products, contract service providers, and technology suppliers to the industry. Topics include process and product development strategies for advanced therapies, regulatory and inspector expectations, automation and closed-system processing, the choice between in-house and outsourced manufacturing, quality assurance and control, analytical methods, viral vectors, and artificial intelligence and Industry 4.0. At the end of each session, presenters…
The Technology of Tomorrow — Today
Sponsored by BioProcess International and its sister publication BioProcess Insider, the “Tech of Tomorrow Zone” at Phacilitate 2020 played host to a number of companies showcasing platforms and ideas that they believe can revolutionize cell and gene therapy (CGT) manufacturing. Some common themes arose in this diverse zone, highlighting technologies from stem-cell supply solutions to viral-vector filling. Participating companies are aware of the complexities involved in producing regenerative medicines, and each proposed solution was intended to reduce the burden on…
April 2020: From the Editor
As I write, the world is in a state of medical and economic uncertainty related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the coronavirus disease (Covid-19) that it causes. Our company group, Informa Connect, has been forced to reschedule some major conferences — including BPI West and BPI Europe — but it’s not alone. Many life-science meetings have been canceled or postponed, and some Biogen employees probably wish their management meeting late in February had been as well.…
Fighting Alzheimer’s Disease with a Breakthrough Peptide
Even more so than cancer, Alzheimer’s disease is one disease that many people fear greatly. The thought of falling prey to the inevitable desecration of our own minds is something that makes even the bravest among us shudder. If we’re robbed of our sense of who we really are, we imagine, then we are doomed to live our last days without the dignity that defines us and that we hold most dear. The ultimate horror of the condition is that…
A Framework for a Competency-Based Curriculum
Training departments in biopharmaceutical manufacturing facilities are at the forefront of ensuring that their employees are trained in accordance with regulatory compliance standards that govern the industry. More important, the purpose of training is to equip employees with relevant knowledge, skills, and abilities to perform their job functions competently. A competency-based curriculum has the potential to facilitate a training approach that addresses both the practical training needs and desired performance outcomes in a workplace. Competency-Based Curriculum Recently, I was involved…
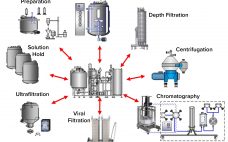
Shared Clean-in-Place Systems: To Share or Not to Share?
Risk of viral contamination is a an accepted part of developing biopharmaceutical products derived from mammalian-cell culture. Viral safety is achieved through a combination of complementary approaches such as selecting non–animal-derived raw materials, testing cell banks, testing for adventitious virus contamination during cultivation, and demonstrating viral reduction capacity of a purification process (1). The latter commonly is referred to as viral clearance by orthogonal purification. Clearly, viral clearance and appropriate viral segregation are important considerations in biopharmaceutical manufacturing process and…
BVDV Risk Mitigation: Dealing with Bovine Viral Diarrhea Virus in Serum
Bovine serum products such as fetal bovine serum (FBS) are critical, nutrient-rich supplements frequently used in cell culture systems for a number of applications, including biotechnology, animal and human pharmaceutical and diagnostic manufacturing, and life-science research. Serum can be contaminated with adventitious agents that could increase its risk for use in cell culture systems. Bovine viral diarrhea virus (BVDV) is one of the most significant infectious diseases in the livestock industry worldwide because of its high prevalence, strong persistence, and…