Emergent BioSolutions is a global life sciences company whose mission is to protect and enhance life. Through our specialty products and contract development and manufacturing services, we are dedicated to providing solutions that address public health threats. Through social responsibility, we aim to build healthier and safer communities. We aspire to deliver peace of mind to our patients and customers so that they can focus on what’s most important in their lives. By working together, we envision protecting or enhancing…
Industry Innovators 2020-2021
Optimal Time to Market for Life-Saving Biopharmaceuticals
Rentschler Biopharma is an international contract development and manufacturing organization (CDMO) dedicated to biopharmaceutical development and biomanufacturing for over 40 years. We are leading experts with an exceptional track record of over 100 different therapeutic protein formats. As a world-class solution provider, we ensure optimum time to proof-of-concept for clinical stages I and 2, as well as time to market for products in clinical stage 3. By accelerating timelines under consideration of economically attractive process options, we generate a competitive…
Biopharmaceutical Development and GMP Manufacturing: Clinical to Commercial Supply
Richter-Helm is a Hamburg, Germany–based contract manufacturing company with a proven 30-year track record and specialized in products derived from bacteria and yeasts. Count on us to flexibly provide a comprehensive range of services and customized solutions. Clients worldwide already have benefited from our commitment to good manufacturing practice (GMP) and total transparency. Our work focuses on recombinant proteins, plasmid DNA, antibody fragments, and vaccines. Our seasoned, 240-strong team supports you with process development, supply of products for clinical trials,…
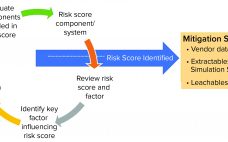
Effective Management of E&L Risk Evaluation to Streamline SUT Implementation
Single-use technologies (SUTs) can introduce leachables (chemicals that migrate from polymeric components into the drug product) to a manufacturing process. Herein, we describe an effective process to manage the risk from leachables to ensure patient safety and prevent delayed product launch. Early implementation of leachables risk evaluation strategies can reduce the resources and time required for risk mitigation later in a project’s life cycle. Fill out the form below to read this technology review now.
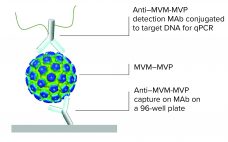
Predicting Viral Clearance at Your Benchtop
Viral contamination is an inherent risk during the manufacture of therapeutic products such as antibodies, vaccines, viral vectors, and plasma derivatives. Whether introduced endogenously from raw materials or exogenously through manufacturing operations, unmitigated viral contaminations can lead to serious health implications and facility shutdowns. Thus, international regulatory agencies require sponsoring companies to validate the “viral clearance efficacy” of their downstream purification process steps before clinical trials or commercial approval. This technology review describes the MockV MVM kit for viral clearance…
High Productivity and Process Economy in GxP Applications with the Octet™ Platform
Using the label-free optical technique of Bio-Layer Interferometry (BLI), the Octet platform provides real-time analysis of molecular interactions. It relies on the robust and easy-to-use Dip and Read™ format, which provides faster time to results relative to technologies like ELISA and SPR. It also operates in a fluidics-free format, thereby minimizing the complexity in analyte detection by fluidics-based technologies like SPR. It provides high-throughput analysis, with the option of analyzing to 96 samples simultaneously, thereby increasing analytical productivity. It has…
Development of a Strategic Quality Control Program for mRNA Vaccines
Messenger RNA (mRNA) therapeutics have the potential to revolutionize several areas of medicine, including the prophylaxis of infectious disease. That potential is driven not only by therapeutic advantages of this modality, but also the relative ease in which a product can be produced and scaled — thus reducing cost and importantly, time to market. mRNA therapeutics have an enhanced safety profile driven in part from their mode of action not requiring integration into the host-cell genome. mRNA vaccines are a…
Bio4C™ ProcessPad: Bioprocessing Data Aggregation and Management, and Near Real-Time Process Monitoring and Analytics
Bio4C™ ProcessPad data visualization, analytics, and process monitoring platform enables bioprocess life-cycle management, reporting, investigations, and continued process verification (CPV). The system intelligently combines process data from disparate sources into a single, validated data source. The Bio4C ProcessPad platform also facilitates 21 CFR Part 11 compliance and ensures access within a validated environment to verified process data that is current and contextual throughout a product life cycle. Fill out the form below to read the complete technology review now.
On the Way to the Optimal Bioprocess
Countless factors influence a bioprocess, and measuring and evaluating them can be a major challenge. One of the most important performance parameters is the volumetric mass-transfer coefficient (kLa). ZETA has developed a reliable method for determining the kLa value and thus has revolutionized bioreactor design in plant engineering. With ZETA’s mass-transfer coefficient value determination system, measurements to determine kLa value can be taken at any point in a bioreactor, and based on such measurements, production conditions can be mapped perfectly.…