Every biomanufacturing process begins with transfection of recombinant genes into pools of cells — followed by a succession of screenings from which will emerge (ideally) a single progenitor cell of the new production cell line. Cast aside will be those cells that do not uptake the correct genetic material, those incapable of thriving in bioprocess conditions, those that fail to produce recombinant protein at relevant levels, and those without demonstrated clonality and relative genetic stability. Over the past several years,…
September 2019 Featured Report
Creating Novel Cell Lines By Genome Editing: Simplifying Cell-Based Assays and Improving Production of Biomolecules
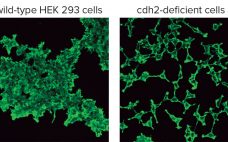
Cultured cell lines have a diverse range of applications. They are used broadly by cell biologists, clinicians, tissue engineers, biotechnology scientists, and bioengineers. The most important uses of cell culture are in the cell-based assays and production of biologically active recombinant proteins. In recent years, genome editing has been used widely to study the structure, function, and localization of endogenous proteins in cultured cells. However, applying the same genome editing techniques to cell lines also could improve the propagation of…
Streamlined Serum-Free Adaptation of CHO-DG44 Cells: Using a Novel Chemically Defined Medium
Monoclonal antibodies (MAbs) have radically transformed the treatment of many chronic diseases, mainly in the fields of oncology and autoimmunity. The overwhelming majority of therapeutic MAbs are manufactured from recombinant Chinese hamster ovary (CHO) cell lines. The original CHO cell line was isolated in the 1950s, and since the early 1980s, it has become the workhorse of the biopharmaceutical industry. The CHO-DG44 strain was generated after several rounds of mutagenesis that deleted both copies of dihydrofolate reductase (dhfr) genes by…