Early manufacturing facilities for large-scale production of biopharmaceuticals were, by necessity, very large. Low expression titers and blockbuster-market products such as monoclonal antibodies (MAbs) combined to require massive bioreactors — with capacities of 10,000– 20,000 L or more — and supporting infrastructure. In my early days covering the industry, I visited a few such facilities and was always awed by the huge tanks and what seemed like miles of piping. A 2010 Pharmaceutical Engineering article described a process modeling approach…
June 2019 Featured Report
Continuous Biomanufacturing: A New Approach to Process Scale
The BioPhorum first-edition Technology Roadmap outlined a 10-year vision for therapeutic protein production in the biopharmaceutical industry (1). The roadmap describes multiple manufacturing scenarios ranging from large-scale (~20,000-L production) to small and agile, portable production facilities. It includes detailed analyses of the needs for the future in each of the following areas: Process technologies (2) Inline monitoring and real-time release (3) Automated facilities (4) Modular and mobile (5) Knowledge management (6) Supply partnership management (7). Since the 2017 publication of…
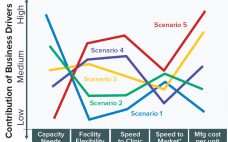
Biomanufacturing Scenarios: From the Biomanufacturing Technology Roadmap
Drug Substance Scenarios Given the complexity of the biopharmaceutical industry and the increasing diversity of products and companies, it is clear that there will be no “one size fits all” solution to biomanufacturing. Instead, we see a range of biomanufacturing scenarios playing out over the next 10 years. Five high-level scenarios were selected for drug substance manufacturing and two for drug product to cover the full spectrum of process and facility types. Each facility type is associated with a representative…
A Shift of Mindset: How Single-Use Systems Influence Bioprocess Engineering and Project Execution
In past decades, the focus in bioprocess engineering was on traditional stainless steel project management, which is highly dependent on established project schedules. Many milestones, tasks, and deliverables during basic and detailed design and execution were implemented in the same tried and tested way by engineering, suppliers, and biopharmaceutical companies. Making decisions was complicated by alignment with long lead items. With the introduction of single-use (SU) technologies, the transition in execution and optimization of project management only just has been…