BioProcess International and the BPI Conference are a bit like twins in that they were born together, but one arrived just ahead of the other. The magazine evolved from a close relationship between its founders and the staff of “IBC Life Sciences” just after the turn of the century. And as we got the publication going back in 2002–2003, we realized that four separate IBC meetings could come together as one under its banner and now-familiar logo. We’ve been colleagues…
September 2017
September Spotlight
“Smaller Is Better” Chemical Bioproduction Ramon Gonzalez, professor of chemical and biomolecular engineering at Rice University and director of its Advanced Biomanufacturing Initiative (iBIO), believes that “waste” methane represents an opportunity for biomanufacturing that should not be missed. Instead of getting burned off, methane could and should generate profits. With advancements in biomanufacturing, wild-type or genetically modified bacteria can turn carbon-rich methane into valuable chemicals — producing them at smaller scales in more environmentally friendly processes than those of classical…
Transforming Deviation Management
All biopharmaceutical companies espouse a belief in scientific, risk-based approaches. However, with respect to deviation management systems (DMSs), the industry is falling short of that promise. By and large, companies still use a small-molecule pharmaceutical compliance model that dates back to the 1980s, based on the strategy that all deviations are created equal and require 30-day closure. Most bioprocessors still hold to a default 30-day rule, even though there is no specific regulatory requirement for that time frame. Major or…
China’s Biopharmaceutical Companies Target Global Markets
Global perspectives of China as a major biopharmaceutical supplier have changed over the past decade. In 2008, BioPlan Associates completed its first analysis and directory of the top 60 biopharmaceutical facilities in China (1). Based on findings from our study titled Advances in Biopharmaceutical Technology in China (2), we found that China clearly held the image of a low quality manufacturer of biogeneric products almost exclusively for its domestic market. Further, concerns over intellectual property protection, contracting problems, and management…
Change Happens: Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management (CMC Forum)
In the current global regulatory environment, management and implementation of postapproval CMC changes often can be unpredictable and inefficient. Timelines for change approval can vary from months to years, depending on regional regulatory procedures. Therefore, the challenge in postapproval lifecycle management is to maintain a constant supply of high-quality product while supporting innovation and continual improvement. This was the premise of the CASSS Chemistry, Manufacturing, and Controls (CMC) Strategy Forum held in Gaithersburg, MD, on 20–21 July 2016. The forum…
The Unican Concept: Engineering Dual Capability into Single-Use Vessels
Use of disposable bioreactors in the biopharmaceutical industry has increased gradually over the past several years in pilot, clinical, and production scale facilities (1–4). Reduced time to market in today’s drug industry has created a need for cost-effective development and production strategies as well as manufacturing flexibility. When compared with traditional stainless steel equipment, disposable bioreactor and mixing systems have smaller space requirements, are portable, and come presterilized to eliminate the need for preuse sterilization procedures such as steam-in-place (SIP).…
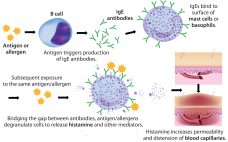
Polysorbates, Biotherapeutics, and Anaphylaxis: A Review
Rapidly increasing use of monoclonal antibodies (MAbs) in the treatment of neoplastic, autoimmune, and inflammatory diseases has led to a dramatic increase in hypersensitivity reactions worldwide, complicating the use of MAbs as first-line therapies and limiting patient survival and quality of life (1). The origins of anaphylaxis are not well understood, though its mechanism is fairly straightforward (Figure 1). It is usually attributed to some undefined intrinsic property or properties of a biotherapeutic — despite the fact that biotherapeutic formulations…
Process Development of Microbial Plasmid DNA: Fast-Tracking with Modular Single-Use Minibioreactors
There has been a rapid rise in the number of positive clinical outputs from clinical studies based on gene and cell therapies. This is in addition to the licensing of products such as GlaxoSmithKline’s Strimvelis ex-vivo stem-cell therapy for treatment of severe combined immunodeficiency caused by adenosine deaminase deficiency (ADA-SCID) in 2016 (1) — has led to an increase in demand for therapeutic vector manufacturing capabilities. Viral vectors are being used for an increasing range of conditions, including monogenetic conditions.…
Insulin Improves Cell Growth and Influenza Yield in a HEK293 Suspension Cell Line
In her “Ask the Expert” webcast on 21 June 2017, Aziza Manceur of the National Research Council Canada (NRC Canada) discussed the benefits of using insulin in bioprocessing. Manceur’s Presentation Canada’s NRC has research facilities across Canada covering disciplines such as aerospace, energy, mining, and medical devices. The NRC serves both the government and the private sector, working with Canadian and international clients. Manceur is based in Montreal with the cell culture scale-up team (in the human health and therapeutic…