Recently, BPI publisher Brian Caine and editor in chief Anne Montgomery had the opportunity to talk with Christel Fenge, Sartorius Stedim’s VP of marketing for fermentation technologies, in the Göttingen, Germany, Sartorius facility. They began by talking about fermentation technology, a topic that led them to touch upon a number of key issues in the biopharmaceutical industry. Fermentation Technology Caine: Because this special issue focuses on fermentation technology, let’s begin by talking about some recent technological improvements and what impact…
2014
Consistently Superior Cell Growth: Achieved with New Polyethylene Film Formulation
During the past decade, single-use bioprocessing bags and bioreactors have gained a significant foothold in the biopharmaceutical industry because they offer a number of advantages over traditional stainless steel equipment, especially for clinical production, multiproduct facilities, and emerging economies. At the same time, some companies are concerned that plastic materials might release potentially toxic substances that could affect cell growth and product titers (1). In a worst-case scenario, they could even compromise drug safety when a company uses disposable bags…
Robust and Convenient Single-Use Processing: The Superior Strength and Flexibility of Flexsafe Bags
With the increased use of disposable bioprocessing bags in all critical process steps of the biopharmaceutical drug production, there is a growing requirement for high-quality, robust, and easy-to-handle bioprocessing bags. The new generation of films and bags must combine multiple mechanical, physical, and chemical properties to make these products suitable and scalable for all processing steps in upstream, downstream, and final filling operations, including cell culture in rocking motion and/or stirred-tank, single-use bioreactors as well as storage, mixing, shipping, and…
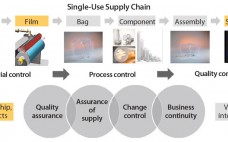
Enhanced Assurance of Supply for Single-Use Bags: Based on Material Science, Quality By Design, and Partnership with Suppliers
Growing adoption of single-use bags in commercial production of biopharmaceutical drugs raises new challenges for bag suppliers and drives the need for consistent product quality, improved assurance of supply, robust change management, and business continuity planning. In close collaboration with resin and film suppliers, polymer scientists and biologists at Sartorius Stedim Biotech have followed a stringent material science and quality by design (QbD) program to develop a completely new polyethylene film and to achieve consistent performance of new Flexsafe bags…
Development and Qualification of a Scalable, Disposable Bioreactor for GMP-Compliant Cell Culture
During the development of single-use, stirred-tank bioreactors (e.g., BIOSTAT STR bioreactors), different phases can be distinguished (Figure 1). First, a clear definition of the intended application and all related requirements should be captured in a user requirement specification (URS). Based on that, the single-use bioreactor design phase and the material selection phase are initiated, both closely linked to each other. During the proof-of-concept phase, relevant component- and product-based tests are established and realized to ensure URS compliance. Finally, the qualification…
Verification of New Flexsafe STR Single-Use Bioreactor Bags: Using a CHO Fed-Batch Monoclonal Antibody Production Process at 1,000-L Scale
In the past decade, single-use bioreactors have gained wide acceptance for biomanufacturing. The biopharmaceutical industry is increasingly interested in performing modern production processes in single-use facilities. That trend is driven by the time and cost benefits of single-use technologies, as well as the enhanced manufacturing flexibility they offer (1). With single-use bioreactors increasingly used in late-phase clinical trials and commercial production, their quality, reliability, and assurance of supply becomes more critical. Many industry experts consider process control of film and…
Pressure Decay Method for Postinstallation Single-Use Bioreactor Bag Testing
Single-use technology is well accepted today, and manufacturers’ quality assurance programs ensure leak-free single-use bags upon delivery. But what about risks involved with installation and other handling errors? Operator training and implementation of suitable standard operating procedures (SOPs) are mandatory, but should they be the only ways to mitigate the risk of failures? In addition, more companies are advocating the use of ballroom concepts (1) for the manufacture of biopharmaceutical drug substances and drug products. However, how do you prove…
Total Solutions Support the Growth of a Dynamic Industry: A Conversation with Reinhard Vogt and Stefan Schlack
While attending a conference at Sartorius Stedim Biotech in Göttingen, Germany, BPI publisher Brian Caine and editor in chief Anne Montgomery spoke with Reinhard Vogt (executive vice president of marketing sales and services, and member of the administrative board) and Stefan Schlack (senior vice president, marketing and product management). They discussed Sartorius’s forward-thinking business strategies, its position as a total solution provider, and how the company’s strategic goals mesh with its assessment of current industry directions. Single-Use As an Enabling…
Disposables for Biomanufacturing: A User’s Perspective
The supply scenario for many biopharmaceutical drugs such as monoclonal antibodies (MAbs) is changing. With the implementation of personalized medicine resulting in drugs for specific, high-responder subsets of patients, market volume per drug will decrease. In addition, increasing fermentation titers of up to 10 g/L for MAbs are leading to smaller fermentation volumes necessary to accommodate individual biopharmaceutical market demands. That results in approaches such as flexible production in campaigns or decentralization of manufacturing using similar facilities with low risk…
September 2014 Issue Author Insights
Exclusive author commentary and article previews for the BioProcess International September 2014 issue.