with Dr. Laura Juckem High-efficiency transient transfections of suspension- adapted Chinese hamster ovary (CHO) cells enable researchers to bridge upstream and downstream processes. This helps to provide confidence that a recombinant protein will have the same properties throughout its development as a biologic. As drug targets become increasingly complex, proper protein folding and posttranslational modifications become more relevant. Early upstream work with the same cell type — e.g., CHO instead of HEK 293 cells — ensures similar product quality from…
November 2014
November 2014 Issue Author Insights
November 2014 Spotlight
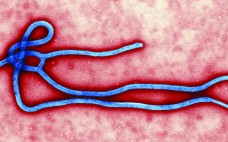
Ebola: Biotech Goes on Counterattack by Cheryl Scott It seems that every year brings a new virus or bacterium to the forefront of the public’s limited attention for infectious disease research. Sometimes it’s a newly identified pathogen such as the severe acute respiratory syndrome (SARS) virus. Often, however, it’s a new strain of influenza or other species — as is the case with this year’s growing focus on the Ebola virus. As recently as March 2014, Reuters was reporting on…
Guidance Is Lacking in the European Biosimilar Regulatory Framework: Considering the Dynamic of Quality Profiles in Development
Biopharmaceutical medicinal products (biologics) had an estimated global commercial market size of US$100 billion in 2013. Because they are more complex than small molecules — and defined by the uniqueness of their manufacturing processes — the generics approval process is not applicable for biologics. Article 10(4) of European Medicines Agency (EMA) directive 2001/83/EC was amended in 2004 in response to the industry’s desire for market access by launching a “similar” biologic abbreviated approval pathway. Leading the subsequent process, the EMA…
Process Effectiveness Analysis Toward Enhanced Operational Efficiency, Faster Product Development, and Lower Operating Costs
Complex drug development and biomanufacturing processes involve back-and-forth shuttling of activities among multiple functions. Close communication, collaboration, and coordination among stakeholder departments and functions are needed to successfully execute these processes. Whereas collaboration between multiple functions leverages each function’s expertise, the resultant structure also poses several challenges, as listed in Table 1 (1). These challenges are further exacerbated as an organization grows in size and geography (2, 3). In the absence of clarity and appropriate assignment of roles and responsibilities,…
Unwanted Immunogenicity: From Risk Assessment to Risk Management
Although vaccines and immunotherapies are designed to engage the human immune system in fighting disease, unwanted immunogenicity can be a major problem for protein-based therapeutics. Some patients produce antidrug antibodies (ADAs), which might lead to drug inactivation or adverse effects. Even human and humanized proteins have proven to be surprisingly immunogenic in some cases, suggesting that immune tolerance requires careful consideration in biologic product design. In rushing to deliver new drugs to market, some biotherapeutics developers have overlooked factors that…
Immunoglobulin Fc-Fusion Proteins Part 2: Therapeutic Uses and Clinical Development
The potential therapeutic value of many proteins — including enzymes, receptors, cytokines, blood factors and peptides — can be realized by fusing them to the Fc region of human immunoglobulin G. Of the 46 monoclonal antibody (MAb) and MAb-derivative products approved by the FDA to date as human therapeutics, 10 are Fc-fusion proteins (Table 2). Among approved products, several structural variations are represented (Figure 4). In BPI’s October 2014 issue, Part 1 of this review examined the structure and manufacturing…
Sterilization Effects on Elastomer Characteristics and Functionality in Parenteral Delivery Systems
To drive efficiencies in producing parenteral drug products, manufacturers are using containers and closure components that are received sterile and ready to be introduced into filling lines. The effects of sterilization on the properties of ready-to-use (RU) components must be assessed to ensure proper processing techniques and suitability over the components’ intended shelf lives. Sterile-drug manufacturers must determine the best sterilization method for components based on their respective drug products and processes. Critical areas of risk include potential changes related…
Reducing Timelines in Early Process Development – Using a Multiparametric Clone-Selection and Feed-Optimization Strategy
The market for biopharmaceutical products remains highly attractive to small biotechnology companies and big pharmaceutical corporations alike (1). Most leading market products are made using recombinant technology (2). Pressures are continually increasing on process development groups to reduce development costs and timelines for taking new clinical products forward from product research bench scale into initial clinical evaluation studies. For many years a recognized critical bottleneck in development of products from mammalian cell lines was selection and isolation of stable, high-producing…
Creating Value Through Investment
During my MBA course, Professor Pierre Casse — then at the International Institute for Management Development (IMD) in Lausanne, Switzerland — regularly reminded us that one key to success was constantly finding new ways to “delight and inspire your clients” by creating value. SAFC achieved that objective in its “Overcoming Supply Chain Vulnerability and Lowering Risk in Biopharmaceutical Manufacturing” symposium 17–18th June 2014 in Turnberry, Scotland. Along with a day of industry insight, the event included a visit and tour…