During the lifecycle of a biopharmaceutical, occasions arise in which the facility and support organization responsible for ensuring that it is manufactured according to schedule, demand, and quality specifications must change, either in whole or in part. The reasons for this vary: some related to scale, some related to clinical development phase, some related to internal manufacturing capacity and program ownership. The industry has adopted the term technology transfer to describe these events. Many such situations have been discussed previously (1,2,3,4). Here, we discuss a scenario in which a product has been bought or licensed by another organization. Our perspective is mostly that of a buyer or licensee. We focus mostly on activities related to chemistry, manufacturing, and controls (CMC) because covering all the aspects of licensing a product in development would be too much for a single article.
With no change in ownership involved, technology transfer commonly occurs under the control of a sponsor company — e.g., in scaling up from a development laboratory to a clinical or commercial manufacturing facility or in transferring a method from one laboratory to another. In such cases, it is relatively straightforward for the sponsor to exert control over all practices and protocols to ensure a robust and successful transfer. Particularly in large corporations, technology transfer may be developed as an important “core competency” within an organization (4). When more than one organization is involved, the resulting challenges are worth examining.
Background
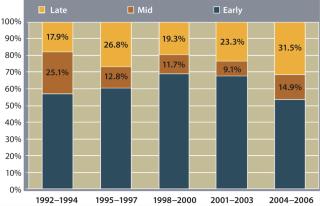
Changes in product ownership are increasingly common and can happen at any stage of a product’s lifecycle. Figure 1 shows an increase in mid-stage and late-stage deals to the highest proportions in a decade, and Figure 2 shows the value of such deals increasing 500% over the same period. The rising values do not in themselves indicate that the volume of deals has increased 500%, but more product transfers are surely part of the picture.
Enabling technology and historical information are transferred along with the rights to develop a product. The degree of complexity usually increases as an active molecule proceeds through its development lifecycle. That complexity makes technology transfer a significant undertaking if it occurs after an investigational new drug (IND) application has been filed. Although tech transfer can occur at preclinical stages, the value of a therapeutic moiety doesn’t seem to build very steeply until it can be demonstrated in humans (5). So the most valuable licensing deals happen after the start of clinical development, at which point an IND — or a even new drug application (NDA) or biologics license application (BLA) — is transferred to a new sponsor organization. ICH Q10 briefly acknowledges the importance of maintaining a system of quality control (QC) over the course of changes in product ownership, and it explains that responsibilities should be defined for both companies, directing management to ensure that the necessary information is transferred (6).
Key elements for successful technology transfer generally include the following:
- Direct involvement and engagement among technical staff on both sides throughout the course of transfer activities
- Well-defined leadership and governance and competent project management
- Multifunctional involvement (matrixed teams) with appropriate competencies on both the transferring and receiving ends
- Meaningful demonstration of success (e.g., a successful good manufacturing practice [GMP] manufacturing campaign resulting in comparable material)
- Good documentation of what is transferred, how it is to be transferred, and the results of that transfer.
A change in product ownership multiplies the challenges of technology transfer. First, the scope is very broad (encompassing not just a set of methods or manufacturing procedures, but all critical information supporting the drug development program to date). Quite often, technology elements applied by a licensor are broad or “platform” technologies that it may be unwilling to part with for any one specific product outlicensing. Untangling all technology ownership supporting a product can often be difficult and contentious in such cases.
Also, the ability of both companies to implement the best practices described herein may be limited. Practices and motivations are usually different among licensee and licensor organizations. Some “translation” of procedures from one organization’s quality system to the other’s will be required. In addition, licensees often have less expertise in at least some aspects of the technology being transferred, and they may in fact transfer it to a third-party organization to execute.
Finally, the desire to recognize transfer-related milestone payments quickly may cause unusual timeline pressures. Those may often coincide with a sharp cut-off of access to the transferring organization’s expertise. To ensure the best outcome for their in-licensed product, the onus is on a licensee to account for such realities in planning and negotiating technology transfer activities.
Although licensees have the greatest stake in making transfers successful and robust, they may be unable to control many elements of transfer processes. Even when best practices cannot be applied, a transfer will still get done, but the outcome is likely to be less robust and/or entail significant additional work (usually by the licensee) to cover gaps or rework processes or methods. This can change the value
of the deal to the new sponsor as well as the overall development timeline. A good licensing agreement will include large incentives for the licensor to ensure that technology transfer is complete and successful. Such incentives could include milestone payments and/or royalties on the marketed product.
Planning and Negotiating Technology Transfer
As mentioned above, “technology transfer” in a licensing situation covers a very broad range of activities in many areas of an organization (manufacturing, development, preclinical, clinical, regulatory, and so on). One part is simply transmitting relevant historical information to each area that gives scientific support for the current state of product development. Failure to have such data can lead to negative regulatory consequences, such as FDA warning letters (7). Clinical-stage products also require transfer of current GMP (CGMP) procedures, processes, methods, and/or materials that must be comparably maintained, controlled, and/or executed by the new sponsor.
Often there are legacy clinical trial materials and ongoing legacy clinical trials, such as compassionate use and treatment IND applications. The participants have a responsibility to continue those trials and serve those patients while the product is developed under the new organization.
Usually a licensee will have a new idea for the product or insight into what caused difficulty for the licensor (thus driving the idea to license the product). In such cases, the drug may be reformulated or its manufacturing process changed to make it more pure, more potent, or less expensive. Such changes must be managed so as to derive the most benefit from existing patient data.
The Importance of Due Diligence: Assessment of technology transfer should be part of evaluation and due diligence activities before a licensing agreement is struck. Due diligence typically focuses on assessing the commercial value of a product to be licensed and assessing whether it is a good strategic fit (5,8). Consider also at this stage whether the new product is a good technology fit. How much expertise do you have in-house relative to the operations used to produce and test the drug? Although lack of in-house manufacturing expertise is usually not a show-stopper, it will affect the value of the deal to the receiving organization, where that expertise will need to be built or hired. With less experience, companies tend to underestimate the difficulty and resources required to ensure a robust transfer of technology (5). So it is a good idea to involve multifunctional team representatives in evaluating a licensing deal.
Intellectual property (IP) considerations should include not only the patent life and rights to the molecule itself, but all associated technologies required for producing and testing it. The associated technologies could affect the licensee’s ability to understand or freely perform certain processing steps, acquire appropriate raw materials, or execute test methods, for example. Aside from arranging secondary licensing agreements, if necessary, you should consider the potential complications that may arise from transferring a process in which certain elements may remain outside of the full control of the licensee.
Licensees should evaluate whether a potentially licensed product has been developed according to current GMP and other regulatory expectations and industry standards. Have appropriate activities relative to each development stage been executed and documented (e.g., process and method characterization or validation)? What is the state of the documentation (batch records, SOPs, regulatory submissions)? Does it adequately cover all development activities (process, analytical, formulation, and so on), and is the documentation readily retrievable for transfer? Ideally it should be comprehensive, systematically organized, and available in electronic formats. Companies also should secure the records of signatures on the transferred documents.
The Contract: As the contractual stage approaches, make sure both parties agree on exactly what technology and information will be transferred and how that transfer will be executed. Depending on negotiated incentives, it may be important to delineate in the contract agreement exactly what information, materials, and equipment will be included. Consider also how you can ensure appropriate involvement of technical experts in the licensor organization. Will you have access to the original development scientists (across the range of relevant disciplines) during transfer of information as well as during subsequent demonstrations of the technology? How much of their time will be available to support these activities?
Here is where you may easily run into conflicts if licensor and licensee have different expectations around the scope of their technology transfer. It is important to understand the relationship between the parties and the incentives on both sides. How involved will the licensor be after the transfer, over what timeframe, and at what cost? Will it have any continuing rights to the product or stake in its success? Is the company’s motivation to simply close the deal and make the product “go away” with the least amount of effort and expense? Because the licensee usually has the most at stake in ensuring success, it may be important to ensure that at least some best practices are specifically agreed upon per contract. Otherwise you’re likely to have trouble getting interest from and traction with the licensor.
What Information Should Be Transferred?
From the licensee perspective, as much information as possible should be transferred. Clearly the licensor will not want to or be able to deliver absolutely every scrap of information or material associated with a product. Some information may not be well documented or may be proprietary, too difficult to retrieve, or considered unnecessary. Some equipment, materials, or proprietary information may need to be retained by the licensor to support other products in its development pipeline. Nevertheless, the licensee should push for as much as possible. Development information may be particularly difficult to obtain, but it may also give you greater technical insight into the product and prevent you from spending resources on repeating work that’s already been done.
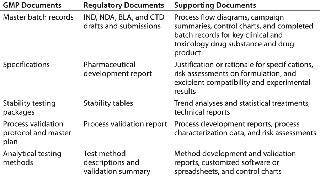
Table 1 lists examples of CMC documents that should be requested. Other items to review include expert reports from third parties or consultants, manufacturing investigations, and out-of-specification (OOS) reports and material safety data sheets (MSDSs) for both drug substance and drug product.
Table 1: Some CMC documents critical to technology transfer
Materials may need to be transferred as well as information, such as
- Previously manufactured drug substance or product (toxicology, GMP materials, primary reference batches) or retains
- Cell banks
- Materials held in stability programs
- Reference and working standards
- Impurities standards
- Assay controls
- Critical analytical reagents (e.g., antib
odies/antigens, primers/probes, and complex matrices) - In-process and/or development retains
- Certain critical raw materials (e.g., single-sourced or proprietary process materials)
- Equipment (particularly, custom or proprietary equipment).
For many of those materials, it may be important as well to obtain associated documentation supporting storage history and relevant chain-of-custody information. Inventory information should be obtained, including applicable expiries. (Expired materials may still be useful for development purposes.) For some analytical reagents, it may be important to obtain qualification protocols and reports, as well as procedures detailing how they were originally manufactured and tested (if, for example, they are proprietary reagents or standards manufactured by the original sponsor). For critical raw materials, it may be necessary to share or transfer previously developed supply chain agreements and relationships. For custom equipment (including software), specifications should be included.
It is worth noting that it is typical to have a number of critical analytical standards and reagents that may not be easy to replenish or replace. One example is a process-specific host-cell protein ELISA (enzyme-linked immunosorbent assay), which may rely on a specific set of antibodies and antigens developed and created by the licensor. Such reagents can actually take years to replace, and no comparable or suitable replacements may be commercially available.
The licensee should ensure that adequate quantities of those analytical materials are transferred (as part of the contract) to support anticipated activities until such time as new sources can be created and requalifed. Keep in mind that activities such as assay transfer and validation — and process development support — can consume many more analytical reagents and reference standards than do routine activities such as release and stability programs. Don’t end up with a situation in which you cannot perform batch release or other critical activities because you lack the appropriate analytical reagents.
Prepare to Accept the Technology
Have a plan for receiving documents that ensures efficient transfer of information. This might include a single point of contact on each end, a tracking system for status and request, a central location for electronic file storage, and a system of organizing received information. You need a mechanism for capturing informal communications during transfer: e.g., questions and clarifications among technical staff of the two companies. Once the transfer is complete, you may have limited or no opportunity to go back to the licensor with additional questions.
Other logistical considerations come up in receiving a new technology. The licensee needs appropriate capabilities to manage and organize information and inventories as well as the appropriate physical facilities for storing and organizing materials. For example, consider how and where to ship and store GMP raw materials, reference standards, stability-tesing materials, cell banks, and so on. Especially where these materials are used to support GMP activities, appropriate control must be ensured over the entire course of the transfer.
If partnered transfer activities include demonstrating a manufacturing process and/or methods in the licensee facility, then that facility’s physical capabilities should be assessed. Technical staff are needed on both sides with competencies in the technologies in use. If a licensee has little experience with the technology being transferred, then its personnel may not ask the right questions, with the result that the transfer is not robust. Licensees should objectively evaluate their expertise in the relevant areas. Those may include manufacturing unit operations, facilities, process chemistry and engineering, and analytics. If expertise gaps are an issue, it is advisable to bring in outside help (expert technical consultants or groups) for support.
During Execution
Good project management practices are extremely valuable in executing a transfer of technology. This includes the development of timelines and objectives, assigning responsibilities, and tracking progress toward agreed-on milestones. To the extent possible, try to build and promote good relationships among team members. Ensure that consistent expectations for supporting transfer activities are communicated across the levels of both organizations (from developing scientists to project managers to executives). If developing scientists are engaged, but their managers are not, then they are likely to be diverted to other projects. If the licensee’s scientists have a “not-invented-here” mindset and resist implementing licensed technology as it was originally developed, then the success of the transfer (as measured by such concepts as product comparability) may be at risk.
A Responsibility Matrix: Common project management tools make sense to use here. A licensee should develop a responsibility matrix. A map of connectivities to all parties should be drawn and maintained because players change during the course of a project. If licensor and licensee motivations conflict, then a steering committee should be empowered to interpret the contract and authorize expenditures to maintain timelines and achieve the goal of the license — that is, to market a product to improve healthcare for patients.
Action items and deliverables should be tracked and tied to specific points on the timeline. If delivery is late, then its timeline impact should be assessed and communicated to the steering committee. If activities are not directed toward points on the timeline, then they should be stopped or the timeline modified to account for the oversight. It is useful for a project manager to update action items off-line, so the group can focus on forward-looking activities and information at a larger scale. The whole group involved in technology transfer should meet regularly to ensure that cross-disciplinary issues are heard by all and to disseminate information on next steps and project status relative to the timeline, budget, and management expectations. However, details and minutiae of specific disciplines should be kept to a minimum and reserved for regular subteam meetings.
Complex technology transfer activities such as demonstration runs should use written protocols: an overarching protocol and/or several smaller ones covering individual activities (such as the transfer of individual analytical methods). Use meaningful, predefined acceptance criteria to demonstrate success. For example, in analytical methods transfer, compare results of materials tested at both the transferring and receiving laboratories. For transfer of a manufacturing process, compare product quality attributes against in-process control limits, historical limits, specifications, and/or other measures. Unlike with internal transfers, contractual obligations can surround the acceptance criteria for such a protocol. A licensor will probably look for broad limits to demonstrate success, whereas a licensee’s interest is better served by narrow limits. Good, transparent, and consistent science is required to set those limits fairly so they will be useful for regulatory purposes as well. Some detailed examples have been published of elements to include in transfer protocols (1).
Use good change-control practices. In some cases, it will be necessary to make changes to a technology as it is transferred from one organization to another. Although it is advisable to minimize changes until transferred technology has been successfully demonstrated by the licensee, some change is unavoidable (e.g., due to differences in manufacturing equipment or operating practices). Any such alterations of a CGMP process should be documented and evaluated with appropriate oversight from the quality and regulatory units of the licensee. Product comparability protocols may
also be advisable for biopharmaceuticals, and demonstration of product comparability is usually a final endpoint for assessing successful completion of the transfer.
Even in the best cases, transfer of technology to a new product owner can result in some loss of historical knowledge. This could be attributable to incomplete or missing documentation or to loss of a gap in expertise or “tribal knowledge” at the new organization. The licensee should attempt to document all known gaps and make plans to address them.
Plan for Success
With any project in biotechnology and pharmaceutical development, planning ahead and doing your homework up front” will pay dividends. Teams that understand the project objectives and timelines, the technology being transferred, and the contractual and regulatory obligations associated with their projects will ultimately do well. Having a preconceived expectation of what data, materials, and equipment will be necessary to conduct a transfer; experts to interpret, use, and operate transferred materials; and places to store, disseminate, archive and retrieve them will allow a licensee to begin replicating the expertise required for successful product development.
Author Details
Dr. Lori Nixon is a senior consultant, and corresponding author Dr. Scott Rudge is a principal consultant at RMC Pharmaceutical Solutions, Inc., 2150 Miller Dr. Suite A, Longmont, CO 80501; lnixon@rmcpharma.com, srudge@rmcpharma.com.
REFERENCES