Freiburg, Germany-based CellGenix says its T-cell medium – CellGenix TCM – offers a serum-free and xeno-free alternative for rapid expansion of functional human T-cells.
Due to stable glutamine in the formulation, the medium is ready-to-use for T-cell cultures without the need for supplementation with human serum or glutamine. Many current T-cell therapy protocols rely on the addition of human serum.
Eliminating the use of human serum will reduce the failure rate in your manufacturing process due to the high lot-to-lot inconsistencies of serum. Since human serum is a limited resource and might not be available in large quantities it is unsuitable for commercial scale manufacturing. Furthermore, the human origin of serum poses a certain risk of containing adventitious agents and therefore does not meet global regulatory guidelines.
Early onset of T-cell expansion and sustained viability
T-cell culture in CellGenix® TCM results in high cell numbers early after activation and throughout cell culture with high viability. This early onset of T-cell expansion allows for faster T-cell therapy manufacturing, which can significantly reduce your cost of goods.
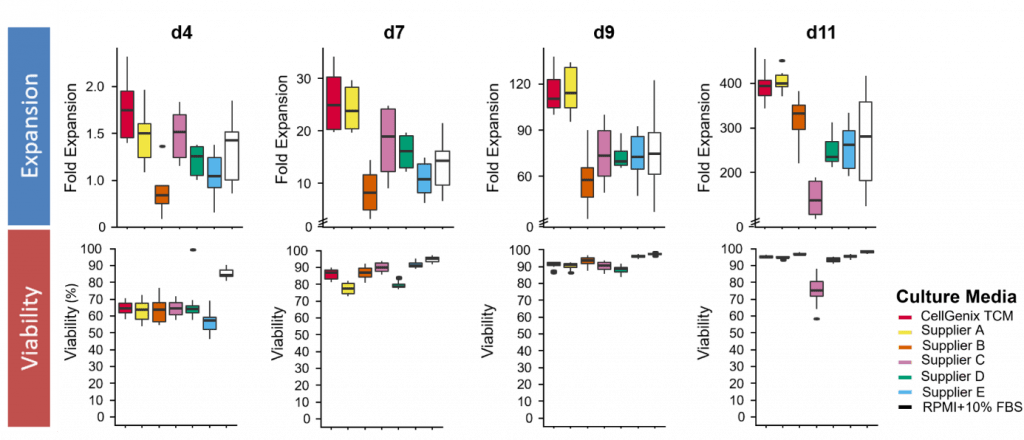
Figure 1: Fold T-cell expansion and cell viability on the indicated day of culture CD3+ T-cells were activated with anti-CD3/anti-CD28-coupled beads and cultivated in CellGenix® TCM or competitor media in the presence of IL-7 and IL-15 (10 ng/ml, CellGenix). On the indicated day cells were labeled with 7-AAD and viability was determined by flow cytometry. Median and spread of n=6 donors are displayed for expansion (upper panels) and cell viability (lower panels).
Promotion of a central memory and early differentiated memory T-cell phenotype
During T-cell culture in CellGenix® TCM a “young” phenotype of naïve T-cells/memory stem T-cells (TN/SCM cells) or central memory T-cells (TCM cells) is acquired. These phenotypes represent the most undifferentiated memory T-cell phenotypes, meaning that they have the highest proliferation potential, self-renewal properties and longer survival rates.
It has been shown that the engraftment and persistence of T-cell products in patients is associated with a TCM phenotype, rather than more differentiated memory phenotypes.
TSCM are considered as even less differentiated cells with potentially even better persistence. It has been reported that for CD19 CAR-T cells the expansion of patient-infused cells is correlated with the frequency of CD8+ TSCM-like cells. It is therefore beneficial for your T-cell therapy product to obtain a central memory and early differentiated memory T-cell phenotype.

Figure 2: Memory phenotype of starting population and after expansion in CellGenix® TCM
CD3+ T-cells were activated with anti-CD3/anti-CD28-coupled beads and cultivated in CellGenix® TCM or competitor media, in the presence of interleukin (IL)-7 and IL-15 (10 ng/ml, CellGenix). On day 0 (left panel) and day 11 (right panel) the expression of memory cell markers was determined by flow cytometry for three donors. TCM, central memory T-cells; TN, naïve T-cells; TSCM, memory stem T-cells; TEM, effector memory T-cells; TEMRA, terminally differentiated effector T-cells.
High proportion of cytokine producing cells including polyfunctional cells
An important aspect of T-cell functionality is the secretion of cytokines such as interleukin-2 (IL-2), interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α). After cultivation in CellGenix® TCM a high proportion of cytokine producing cells is obtained including polyfunctional cells (expressing more than one cytokine).
A recent report suggests that the combination of cell frequency and cytokine production levels of polyfunctional CD19 CAR-T cells is correlated with the clinical outcome in patients with non-Hodgkin lymphoma. Obtaining cytokine secreting T-cells, in particular polyfunctional cells, could therefore have a positive effect on the therapeutic response of your T-cell therapy product.

Figure 3: Cytokine expression after expansion in CellGenix® TCM
CD3+ T-cells were activated with anti-CD3/anti-CD28-coupled beads and cultivated in CellGenix® TCM or competitor media in the presence of IL-7 and IL-15 (10 ng/ml, CellGenix). On day 11 of culture, cells were re-stimulated using phorbol 12-myristate 13-acetate (PMA) and lonomycin in the presence of brefeldin A. Cells were fixed and permeabilized and the expression of the indicated cytokines was determined by flow cytometry. Median and spread of n=6 donors are displayed for CD4+ (upper panels) and CD8+ (lower panels), black dots indicate statistical outliers.
