Removal of host cell protein (HCP) impurities in drug substances is critical in the manufacture of highquality drug products and for patient safety. A key requirement is a thorough analysis of HCP contaminants, which may vary considerably depending on cell line, media, and process parameters. How well a particular HCP assay recognizes all HCPs will depend on how well its antibodies match the actual HCP composition, their abundance, and affinities for each HCP. Accordingly, various commercial generic HCP ELISAs may…
Author Archives: Yvonne Haberkorn
New Host-Cell Protein Assay for Escherichia coli Cell Lines
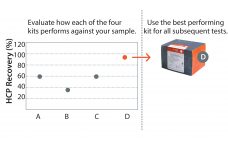
With the E.coli|360-HCP assay, a second host-cell protein (HCP) assay based on the novel 360-HCP ELISA concept enters the market. 360-HCP ELISA integrates four different assay types. So a scientist can evaluate in no time which kit type performs best for a specific process. Read the full text of this application note in the PDF (Login required).
A New Generation of Generic HCP Assays
Host-cell protein (HCP) analysis is a big issue when it comes to early clinical testing, design of downstream processes, and quality control of biopharmaceuticals. The decision to use an off-the-shelf generic HCP assay or spend a considerable amount of money for development of a specific HCP assay is often not an easy one. In theory, generic HCP assays should be suitable for all HCP determinations of a specific cell line — independent of cell line modifications, fermentation conditions, and the…