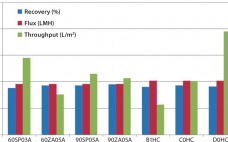
Since the early 1980s, biotechnology products have been a fast-growing sector. They now occupy a significant portion of biologic drugs approved by regulatory authorities around the world every year. Among the approved biologic drug products, as well as those still in clinical testing, many are manufactured by contract manufacturing organizations (CMOs). Sponsor companies often transfer their developed process and process knowledge to CMOs for manufacturing of materials for toxicology and clinical studies. Drug Product Development Figure 1 illustrates the usual…
Thursday November 06, 2025