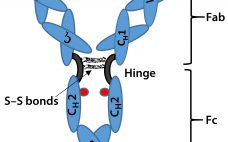
Last month, Part 1 of this discussion briefly described the regulatory landscape for developing biosimilar therapeutic monoclonal antibodies (TMAbs). We identified certain specific structural components of TMAb drug substances that warrant particular attention because alterations to them are likely to affect therapeutic safety and effectiveness. Now we conclude by considering whether studies of reference materials can further the development of analytical industry standards to ensure comparability of putative biosimilar TMAbs with innovator TMAbs. We suggest that the time is right…
Author Archives: William E. Bentley
Biosimilar Therapeutic Monoclonal Antibodies: Gaps in Science Limit Development of an Industry Standard for Their Regulatory Approval, Part 1
Biosimilars are biologically derived pharmaceuticals intended to have clinical similarity to a legally marketed innovator product when that product’s patent or market exclusivity has expired. By contrast with generic small-molecule drugs, clinical performance of a biologic pharmaceutical is a function of its structural complexity and higher-order structure (HOS). Biomanufacturing controls of such complex products cannot fully ensure chemical similarity between an innovator product and putative biosimilar because minor differences in chemical modifications and HOS can significantly alter a product’s safety…