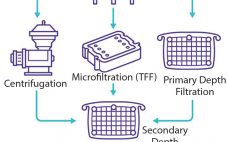
A general vaccine purification strategy can be divided into three stages, with one or more steps for each stage. The first stage is to concentrate and isolate the target molecule quickly to remove it from conditions that could lead to its inactivation or loss. Intermediate purification seeks to remove remaining contaminants, typically using an orthogonal approach. That is followed by a polishing step in which trace impurities are removed through high-efficiency steps because those impurities usually are similar to the…
Author Archives: Takao Ito
Filter-Based Clarification of Viral Vaccines and Vectors
Viral vaccines rely on the antigen properties of a virus or virus-like entity to trigger an immune response and induce immune protection against a forthcoming viral infection. Through development of recombinant viral vaccines, developers can reduce risks associated with the presence of live and inactivated viruses. Instead, recombinant vaccines induce immunity against a pathogen by relying on the capacity of one or more antigens delivered by means of viral vectors or the baculovirus/plasmid system (1). Viral vaccines are formulated with…
Pressure Rating for Bioprocess Single-Use Assemblies
Single-use systems (SUSs) are engineered process equipment solutions for pharmaceutical and biologics production. They offer several key advantages, such as lowering energy costs to reduce utility requirements, minimizing cleaning validation efforts, reducing water and chemical use, and enabling flexibility in manufacturing. Use of SUSs is increasingly popular in almost all fields of bioprocess applications (1, 2). SUSs most commonly comprise components made of polymeric materials, which together create a system or unit operation designed for one-time or campaign use. Single-use…