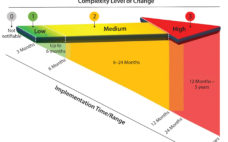
Current practices for change notification in the biopharmaceutical industry are neither efficient nor conducive to accelerating the adoption of single-use systems (1, 2). Drug manufacturers (end users) often observe that supplier change data packages lack technical content or detail and that the time allowed for change implementation is too short. Occasionally, customers (end users or next tier in supply chain) learn of changes after the fact, possibly even by happenstance. Suppliers, however, can find the potential affect of a change…
Sunday October 26, 2025