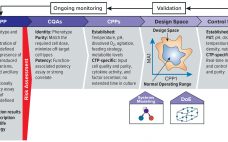
As advanced therapies, including regenerative medicines, progress toward commercialization and market approval, early warnings from key opinion leaders (1, 2) regarding the importance of better understanding quality target product profiles (QTPPs) and critical quality attributes (CQAs) of such products have resounded ever louder (see the “Terminology” box for definitions). Costly late-stage delays, redirections, and even abandonment of clinical programs can be linked to quality issues associated with inadequate understanding of process and product. Therefore, a review of the benefits of…
Saturday October 25, 2025