Manufacture of biopharmaceuticals using mammalian cells inherently incurs a risk of viral contamination during cell cultivation. If introduced, viruses can infect and replicate in cells used to produce a therapeutic protein or vaccine. The consequences of such contaminations can be dramatic. Not only can a company lose contaminated batches, but it also faces potentially extensive root-cause investigations, facility cleanup efforts, and introduction of preventive measures. Until contamination issues are resolved adequately, production should not be resumed, and facility downtime brings…
Author Archives: Per Kærsgaard
How Much Harm Can a Single Droplet Do? Considerations for a Viral Inactivation Step
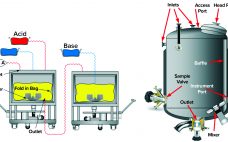
Viral clearance is a fundamental aspect of viral safety for biopharmaceutical products. Regulatory agencies around the world require biomanufacturers to segregate their operations appropriately to mitigate the risks of carryover contamination from previous process steps or product batches and of crossover contamination between product(s) made in the same facility. Guidelines are vague in defining “appropriate,” leaving biomanufacturers to interpret regulatory expectations and define their own virus reduction and segregation strategies. Given the differences among manufacturing processes and facilities housing such…
Virus Segregation During Purification Processes: Calculation of Critical Potential Carryover of Viruses
Before a pharmaceutical product is introduced into humans, either in a clinical trial or as a marketed product, virus safety must be evaluated carefully. Virus safety normally is ensured using a three step complementary approach: selecting and testing cell lines and/or raw materials for the absence of viruses, testing the product at appropriate steps of production, and assessing the capacity of a production process to clear infectious viruses (1). The latter (also referred to as viral clearance) is the subject herein. Spiking studies are conducted to evaluate the capacity of a purification…