Continuous biomanufacturing was a central topic at the fourth annual World Biological Forum in Oxford, UK, on 26–28 June 2017. A well-rounded lineup of presenters appeared at this forum held in Oxford University’s Lady Margaret Hall, an eclectic location that well captured the historic charm of the university. Delegates were well supported throughout the meeting with generous meals, refreshments, and assistance provided by helpful staff. Papers were presented in Talbot Hall in the center of the college. The stately main…
Author Archives: Morten Munk
The Industry’s Hesitation to Adopt Continuous Bioprocessing: Recommendations for Deciding What, Where, and When to Implement
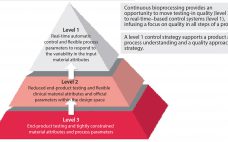
The US Food and Drug Administration has stated its appreciation of continuous bioprocessing (CBP), and some studies have shown that it can save manufacturers time and money. However, the bioprocessing industry is still reluctant to implement continuous bioprocessing right away. It will be interesting to see which companies will be among the first-movers to harness the competitive benefits. Although few biologics today are made using CBP-enabled equipment (e.g., advanced bioreactors), the industry is changing. For biologics already in production, it…
Meeting the Demand for a New Generation of Flexible and Agile Manufacturing Facilities: An Engineering Challenge
Classical stainless steel installations in purpose-built facilities dominate the global capacity for commercial biopharmaceutical manufacturing. Early facilities that were designed for single-product processes are now aging, putting them on the investment radar for upgrades to enable manufacturing diversity, and allow more efficient facility use. More than ever before, global engineering leaders are confronted with complex strategic and financial decisions when they seek to invest capital in new flexible pharmaceutical facilities or flex-grading aging facilities for supply of pipeline products. Manufacturing…