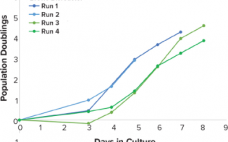
With the increase of T-cell immunotherapy research, cell therapy developers have encountered the difficulty of producing consistent, high-quality products. Approved therapies Kymriah™ (tisagenlecleucel, Novartis), Yescarta™ (axicabtagene ciloleucel, Kite Pharma), and late-phase clinical products BB2121 and UCART19 have been critiqued for presenting potential obstacles to patient access because of high costs of treatment and variable manufacturing outcomes. Manufacturing processes need to be developed to increase control over each unit step in a way that improves product consistency over the life of…
Sunday September 14, 2025