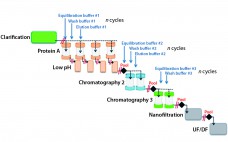
Process intensification through continuous manufacturing has been practiced in the chemical, petrochemical, and food industries for years and has gained much interest among biopharmaceutical manufacturers (1). Key drivers encouraging biomanufacturers of therapeutic molecules to convert batch processes into continuous operation include flexibility, productivity, cost effectiveness, and product consistency. Continuous upstream processing has been demonstrated for the manufacture of a broad range of molecules, including complex/labile proteins such as enzymes (2) and monoclonal antibodies (3). Recent publications have reported successful application…
Monday October 27, 2025