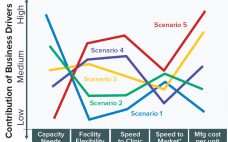
Drug Substance Scenarios Given the complexity of the biopharmaceutical industry and the increasing diversity of products and companies, it is clear that there will be no “one size fits all” solution to biomanufacturing. Instead, we see a range of biomanufacturing scenarios playing out over the next 10 years. Five high-level scenarios were selected for drug substance manufacturing and two for drug product to cover the full spectrum of process and facility types. Each facility type is associated with a representative…
Author Archives: Linda Wilson
BioPhorum Operations Group Technology Roadmapping, Part 3: Enabling Technologies and Capabilities
Although great strides have been made over the past 20 years to increase the productivity and robustness of manufacturing processes for biopharmaceuticals, the cost and complexity of their development and manufacturing remain high, especially in comparison with those of small-molecule pharmaceuticals. Process improvements are required to increase patient access while maintaining the viability of an R&D-driven biopharmaceutical industry. Facility productivity, cost of goods (CoG), and capital investment all have significant margins for improvement. Such goals can be achieved not only…