On Thursday 6 September 2018 at the annual BioProcess International Conference in Boston, the first “Technology Round Robin Featuring Six Innovative Bioprocess Technologies” was presented in an interactive session with attendees as active participants, asking questions and engaging in conversation with the six featured entrepreneurs. Detailed below, this session was a culmination of several steps in an overall strategy for some of the companies participating. To fully appreciate the launch of new technologies into the bioprocess arena, you first must…
Author Archives: Lynne Frick
Driving Cell Therapy Innovation: Applying Key Lessons from the Evolution and Commercialization of Protein-Based Therapies
After many trials and errors — and milestones — regenerative medicine has become a mainstream part of the biopharmaceutical industry, supported by at least 670 companies and clinics of all sizes. But many experiences in the protein-based industry segment can be leveraged to further improve successful commercialization of advanced therapies. At the 2017 Biotech Week Boston conference, BioProcess International editor in chief Anne Montgomery hosted a panel of industry cell therapy experts to discuss key lessons that can be gleaned…
Integrated and Scalable Continuous Chromatography: The Cadence BioSMB PD System from Pall
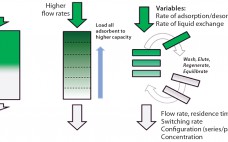
Bioprocess intensification leads to more efficient, flexible, and cost-effective biopharmaceutical manufacturing facilities. Single-use systems designed for continuous operation facilitate process intensification. Pall Life Science’s Cadence™ BioSMB PD platform is the first disposable flow path, continuous multicolumn chromatography solution that is fully scalable from a process development laboratory to good manufacturing practice (GMP) manufacturing. It provides an economically viable, disposable chromatographic solution for even the most demanding and costly chromatographic steps. During multicolumn countercurrent (also known as simulated moving bed, or…
Continuous Chromatography Is Now Possible for Clinical Manufacturing
Intensified and integrated bioprocess technologies are creating a paradigm shift toward more efficient, higher flexibility facilities for biopharmaceutical manufacturing. Continuous technologies that are designed as single-use systems help to greatly facilitate process intensification, delivering further efficiencies with reduced set-up times and elimination of the need for cleaning and cleaning validation. Chromatography is often considered to be a challenging bioprocess step, which has caused great interest in a simplified, safer solution. Continuous multicolumn chromatography using a single-use flow path is an…