Patients receiving particulate contamination through parenteral delivery of biopharmaceuticals presents a significant potential health risk. However, the severity of that risk often is unclear. It depends on the route of administration, dosage volume administered, particle properties and amount received, and the ultimate fate of particles within a patient’s body (1). The appearance of particulate contamination also can be a visible indicator of product quality. Consequently, when such contamination is discovered within biopharmaceutical manufacturing operations, often it triggers costly investigations and…
Author Archives: James Vogel
An Industry Proposal for Change Notification Practices for Single-Use Biomanufacturing Systems
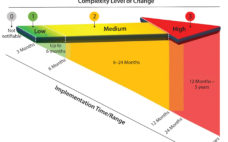
Current practices for change notification in the biopharmaceutical industry are neither efficient nor conducive to accelerating the adoption of single-use systems (1, 2). Drug manufacturers (end users) often observe that supplier change data packages lack technical content or detail and that the time allowed for change implementation is too short. Occasionally, customers (end users or next tier in supply chain) learn of changes after the fact, possibly even by happenstance. Suppliers, however, can find the potential affect of a change…
Coordination of Single-Use System Standards and Best-Practice Efforts
A BPI Theater Roundtable at Interphex 2016 On Tuesday, 26 April 2016, James D. Vogel (founder and director of The BioProcess Institute at the University of Rhode Island) chaired a midday roundtable titled, “Single-Use Harmonization Town Hall: Coordination of SUS Standards and Best Practice Efforts.” Two industry experts joined him in a panel discussion: Mike Johnson (business development engineering manager for Entegris) Jeff Carter (strategic projects leader at GE Healthcare). All three are members of different groups interested in developing…
Utilizing Environmental Monitoring to Ensure Facility State of Control
James D. Vogel (founder and director, The BioProcess Institute) BPI Theater @ INTERPHEX, April 27, 2016, 11:40 am–12:00 pm James Vogel introduced the concept of trending and how to use it to improve a manufacturing facility’s performance. Trending is defined as collecting data and then examining that information for trends — for example, modeling data to forecast the weather. Sometime a simple graph tells you as much as more advanced statistics can. The key is to pay attention to present…