Gene therapy is one of the most progressing fields in life sciences. Although only eight gene therapy products have reached market approval, the pipeline is robust with ~500 candidates in different stages of clinical development. More than 1,700 clinical studies are being conducted, and the value of this industry segment is expected to grow by ~17% annually over the next 10 years. Gene therapy involves using genetic material to fight or prevent disease. The gene is delivered into a patient’s…
Author Archives: Josefina Nilsson
Viral Vector Particle Integrity and Purity Analyses in Early Process Development
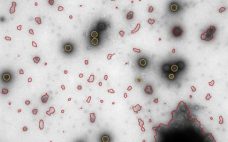
Gene therapy is the transfer of genetic material to a patient’s cells to achieve a therapeutic effect. Therapeutic DNA typically is delivered using a viral vector system, and adenoviruses have been used for this purpose for over 20 years (1–3). Within the past 10 years or so, lentiviruses have shown promise in clinical trials (1–3), and adenoassociated viruses (AAVs) have been used in the first approved gene therapies in the Western world (4). The number of gene therapy applications based…
Reveal Information That Provides Insights: New Approaches to Subvisible Particle Characterization
In an October webcast, Vironova’s Josefina Nilsson (head of electron microscopy services) and Gustaf Kylberg (MiniTEM product manager) discussed subvisible particle characterization using the MiniTEM system, which provides both morphology and quantitative data. Nilsson and Kylberg’s Presentation Vironova combines expertise in electron microscopy and image analysis with in-house designed software. Electron microscopy is a good technique for getting to know your product in detail (morphology and other characteristics). After discussions with partners and clients, Vironova worked with Delong Instruments to…