Protein A has been a fantastic tool for the antibody industry. It is without doubt the most well-established purification technique in monoclonal antibody (MAb) manufacturing today due to a few key success factors. Protein A is the result of the long evolution of Staphylococcus aureus that developed a defense system against antibodies. Protein A exists on the cell wall of about 9% of S. aureus strains and immobilizes IgGs. When there is an immune response in the body, the bacterium…
Author Archives: Jonathan Royce
Reimagining Capacity for Today’s Purification of Monoclonal Antibodies
The monoclonal antibody (MAb) market has grown over the past decade to be about half of the biomanufacturing market today. This growth should continue, driven by a strong pipeline of MAbs that are currently in phase 2 and 3 clinical trials. A synergistic evolution of MAbs and protein A resins also has taken place in the market. Figure 1 shows a graph adapted from an Amgen study of the development of protein A productivity and capacity over the past 40…
An Industrial Platform Solution for Antibody Fragment Purification
Compared with traditional approaches such as chemotherapy and radiotherapy, monoclonal antibodies (MAbs) have become the most successful cancer treatments in the past 20 years (1). With great clinical success in many therapeutic areas, MAbs now account for >40% of the entire biotechnology drug market, and sales are projected to be >US$160 billion over the next few years in the United States alone (2). More than 35 MAbs have been approved for clinical use, and hundreds more are filling industry development…
High-Capacity Protein A Chromatography Medium for MAb Capture from High-Titer Feeds
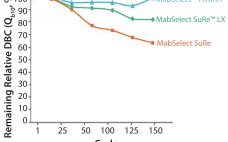
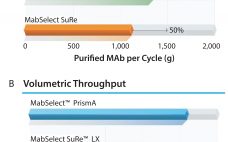
As product titers continue to increase in upstream cell culture processes, so too the demand for enhanced capacity in downstream purification steps. MabSelect SuRe LX chromatography medium (resin) is specially developed for monoclonal antibody (MAb) capture from high-titer feeds. To help ensure good process economy, the medium can withstand hundreds of rigorous cleaning cycles with NaOH, with retained dynamic binding capacity (DBC) and MAb yield. High Dynamic Binding Capacity (DBC) Large-scale purification of MAbs usually consists of two or three…
Development of a Turn-Key Harvest Solution for Small-Volume Bioreactors
Over the past 10 years, disposable bioreactors have grown from a niche tool servicing small-scale projects to a common and essential component in the CGMP production of human therapeutics (1). Recent advances in filter integration, aseptic connectors, and disposable sensing allow entire cell culture processes to be performed using only single-use components. However, harvest and clarification operations remain largely dependent on centrifugation, cross-flow filtration, and depth filtration (2), which are all techniques that have not been widely adapted to single-use…