As in most high-tech sectors, success within the highly regulated biopharmaceutical manufacturing industry depends upon an adaptable, skilled, educated, and high-performing workforce. The impact of human performance cuts across the entire industry — whether for a manufacturing operator, a scientist or engineer in a highly technical role such as process development, or a technical sales representative serving the industry. The effects of poor performance can be seen easily in deviation reports and FDA warning letters as well as in quantified…
Author Archives: John Balchunas
Measuring the Impact of Investments in Professional Development: A Virtual Roundtable
Well-designed education, training, and professional development programs and partnerships add considerable return on investment to stakeholders across the biopharmaceutical manufacturing industry. As biomanufacturers, suppliers, and regulatory bodies share similar workforce needs, focused education and training efforts can lead to increases in productivity, yield, and employee engagement and retention. In addition, education and training programs can decrease the incidence of deviations, which are costly to investigate and remediate. Yet when compared with other business needs, training, development, and human performance initiatives…
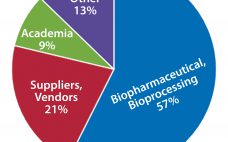
Demographics and Trends: Results from a Joint BPI/BTEC Survey on Training
Training is an investment. And it’s one that the authors, contributors, and other individuals behind this supplement understand well. However, the biopharmaceutical industry is much larger than these few individuals. It’s just as important to understand the motivators, drivers, and training-related challenges faced by the industry at large. So BPI and BTEC jointly administered a short survey to gather some basic information from the magazine’s readership about the value and role that training plays in their organizations. According to survey…