NeoStem, Inc. November 4, 2013 New York, NY. NeoStem, Inc. (NASDAQ: NBS), a leader in the emerging cellular therapy industry, and its subsidiary, Progenitor Cell Therapy, LLC (“PCT”), an internationally recognized contract development and manufacturing organization, announced today that PCT has entered into a collaboration with ATMI, Inc. (NASDAQ: ATMI), a global technology company and leader in single-use bioprocess solutions. As industry leaders, PCT and ATMI will collaborate on a non-exclusive basis enabling PCT and PCT’s affiliates to offer to…
Author Archives: BPI Contributor
Analytical Advances: The Power of Mass Spectrometry
This month, Lester Taylor, director of LC-MS product marketing, and Ning Tang, senior application scientist (both at Agilent Technologies) talk about the advances that have extended adoption of mass spectrometry (MS) in QA/QC laboratories. Ideal technologies for analyzing large proteins and peptides include high-resolution LC-MS time-of-flight (TOF) spectrometry and hybrid LC-MS quadrupole TOF (Q-TOF) methods. The experts also discuss software tools for MS analysis, which facilitate the processing of complex data sets. Traditional biopharmaceutical applications for MS include protein molecular-weight…
Delivering Affordable Biologics from Gene to Vial
Miriam Monge (from Biopharm Services) revisits her March 2010 article (coauthored with Andrew Sinclair) in an audiocast with managing editor Maribel Rios. She reviewed the industry’s economic challenges at that time, which included costs associated with treatment, increasing market competition, and the rising demand for approved biosimilar products to offset healthcare costs. They wrote, “Many issues the biopharm sector faces appear to be the consequence of a maturing industry with structural impediments to its development brought about by regulation, political…
Monoclonal Antibody Purification with a High Capacity Protein A Resin
“Protein A resins constitute a substantial cost in state-of-the-art mAb purification processes. Factors such as operating cycles, capacity, and mAb titer can have an impact on total costs associated with mAb purification. The purification of a monoclonal antibody from crude feed stock using TOYOPEARL® AF-rProtein A HC-650F, a high capacity protein A resin from Tosoh Bioscience, show that this particular high capacity protein A resin delivers highly pure antibodies at yields approaching 90%.”
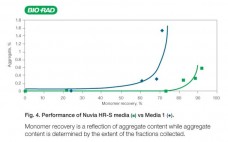
Introducing Nuvia HR-S Chromatography Media
Nuvia HR-S media is a new strong cation exchanger that has been optimized for particle size and chemistry that provides exceptional resolution and high recovery. Nuvia HR-S media demonstrates fast mass transfer kinetics, excellent flow characteristics, and robust chemical stability against common caustic cleaning protocols. Its excellent scalability gives process developers the confidence that results obtained on the bench will be reproducible for large-scale downstream manufacturing. Nuvia HR-S media is the preferred solution for intermediate and final polish applications where…
TOYOPEARL AF-rProtein A HC-650F Host Cell Protein Removal
The following paper compares the host cell protein (HCP) removal capabilities of TOYOPEARL AF-rProtein A HC-650F, TOYOPEARL AF-rProtein A-650F, and another commercially available high capacity protein A resin.
An environmental life cycle assessment comparison of single-use and conventional bioprocessing technology
Single-use technologies offer an attractive option for biopharmaceutical manufacturing, but their environmental impact needs be considered. This paper documents the findings of a Life Cycle Assessment (LCA) study comparing single-use and conventional bioprocessing technology for the production of monoclonal antibodies (MAbs). The study examines the life cycle environmental impacts at 100 L, 500 L, and 2000 L. The results presented focus on the 2000 L production scale. This assessment was reviewed by a third-party review panel. The results demonstrate that…
Six Essential Workflow Steps for Paperless, Automated, End-to-End Environmental Monitoring
The consequences of microbiological contamination range from FDA warning letters through product quarantines and recalls all the way to plant shutdowns. This makes quality control, specifically microbiological and environmental monitoring (EM) a mission-critical priority for pharmaceutical and biotechnology drug manufacturing operations.
Are you currently struggling with integrating a home-grown, paper-based or semi-automated EM system to your LIMS only to find out that it’s error-prone and resource hungry? Are your current LIMS or IT systems over extended when it comes to customizing automated, paperless, mobile data capture and workflow management for EM? Are you searching for a proven, best-practices approach to automate EM and LIMS workflows and reporting in a cGMP environment without hindering new product development and production?
This whitepaper provides an overview of the essential workflow steps for implementing a paperless, automated, end-to-end environmental monitoring solution to increase productivity, reduce compliance risk and generate a healthy return on investment.
November 2013 Issue Author Insights
DSM Celebrates Opening of New cGMP Facility for Biopharmaceutical Manufacturing in Brisbane, Australia
DSM Pharmaceutical Products DSM Pharmaceutical Products, the custom manufacturing and technology business of Royal DSM (NYSE, Euronext: DSM KON), today officially opens its new cGMP facility for biopharmaceutical contract manufacturing in Brisbane, Australia. The facility was built in partnership with Biopharmaceuticals Australia with cooperation from the government of Queensland and the Commonwealth of Australia. This world-class operation in the Asia-Pacific region is an important growth area in DSM’s strategic development in the biopharmaceutical field. The facility was designed by an…