GE Healthcare Life Sciences, a provider of tools, technologies and services to the biopharmaceutical manufacturing industry, and Rentschler Biotechnologie GmbH, a leading contract manufacturing and development organization (CDMO) for biopharmaceuticals, announced today a joint celebration to mark the handover of GE Healthcare’s 1000th Ă„KTAprocess™ and the expansion of Rentschler’s biopharmaceutical manufacturing capabilities in Laupheim, Germany. Ă„KTAprocess, GE’s automated process and manufacturing scale chromatography system, has gone into operation as part of Rentschler’s recently expanded single-use manufacturing suite. GE biomanufacturing technologies…
Author Archives: BPI Contributor
Sartoclear Dynamics®: Single-Use Harvesting Technology for High Cell Density Cultures up to 2,000 L
Sartorius Stedim Biotech (SSB), a leading international supplier for the biopharmaceutical industry, has introduced Sartoclear Dynamics®, a clarification system featuring new single-use technology for harvesting mammalian cell cultures with high cell densities. Continuous improvements in growth media and cell lines have elevated biomass concentrations in bioprocesses. Therefore, these increasingly higher concentrations place growing challenges on the purification process. As body feed filtration has proved to be the best solution to solve similar challenges in related industries, Sartorius Stedim Biotech has…
SAFC Launches New EX-CELL® Advanced™ Products for Scalable, Efficient Biopharmaceutical Manufacturing
Sigma-Aldrich Corporation today announced that SAFC® Commercial, the Company’s business unit providing products and services for use in regulated pharmaceutical and biopharmaceutical applications, has launched the EX-CELL® Advanced™ product line. Designed to address the needs of an evolving industry where speed to market is paramount, the new product line provides for increased performance, streamlines regulatory compliance, and offers the supply chain security needed in today’s biopharmaceutical environment. The first EX-CELL Advanced product is the high-performing batch media system developed for…
A Xeno-Free Culture System for hMSC from Various Sources Suitable for Initial Isolation and Expansion Toward Clinical Applications
Human mesenchymal stem cells (hMSC) are multipotent adult stem cells present in a variety of tissue niches in the human body. hMSC have advantages over other stem cell types due to the broad variety of their tissue sources, since they are immuno-privileged, and for their ability to specifically migrate to tumors and wounds in vivo. Due to these traits hMSC have become desirable tools in tissue engineering and cell therapy. In most clinical applications hMSC are expanded in vitro before…
CaPure-HA™: DoE Optimization of Elution Conditions
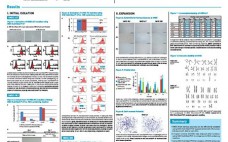
Design of Experiments (DoE), also called experimental design, is a statistical approach to process development that has gained wide acceptance in the biopharmaceutical industry. DoE is used to reduce development costs by speeding up the design process and to optimize the parameters of a particular step in the manufacturing process. The data presented here demonstrate the optimization of the CaPure-HA elution using a DoE approach.
Pressure Interruptions (Stop/Start) During Virus Filtration: Assuring Safety Using Robust Process Technology and An Appropriate Risk Mitigation Strategy
Following publications discussing the impact of pressure interruptions on the retention levels of virus filters, an increased focus is being placed on filter users’ own risk mitigation strategies. Users are increasingly requesting technical guidance and support with viral validation from filtration experts, as part of their overall product support package. This guidance document provides a high level of assurance to filter users by demonstrating how combining robust virus clearance technology with the use of standard operating procedures can assure a…
Sterile Vent Filtration on Ozonated Water Tanks
With many pharmaceutical processes requiring large volumes of water, it is critical that any pharmaceutical-grade water used is protected from particulate or microorganism contamination, in order to ensure that process operations do not become inadvertently contaminated. Several approaches can be used to ensure that water remains free from contamination, including the storage of purified water or WFI (Water For Injection) at a minimum temperature of 80°C to discourage microbial growth in the storage system. Another approach is to add ozone,…
SPD Vent Option for UltraCap® H.D. Capsule Filter
Meissner’s Sanitary Process Design (SPD) vent option has been purpose designed for use within single-use systems applications. The vent features a full size 1/8” hose barb, compatible with tubing and affixment methods typically used in single-use systems, to support robust closed system sampling operations. The SPD vent’s large knurled grip allows it to be easily manipulated by gloved hands. Multiturn flow initiation provides precise metering during operations where venting is taking place to a closed system, (e.g. a biocontainer). Meissner…
GEA Niro Soavi Announces the Ariete NS5180 Homogenizer
GEA Niro Soavi has developed the Ariete NS5180 homogenizer for powerful and reliable high pressure with easy maintenance and cleaning. Compliant to EU safety rules (CE standards) and built according to EN ISO 9001:2008 Quality Systems, the Ariete NS5180 homogenizers and high pressure pumps have all the technical solutions for any processing need. GEA Niro Soavi’s Ariete NS5180 homogenizers are constructed of high quality stainless steel and corrosion resistant materials and are available in a wide range of high pressure…
EMD Millipore Granted Patent for Selective Layering Method that Improves the Consistency of Virus Filtration Performance
EMD Millipore, the Life Science division of Merck KGaA of Darmstadt, Germany, has been granted a United States patent for developing a selective layering method that significantly improves the consistency of virus filtration performance. The method is used to manufacture EMD Millipore’s Viresolve® Pro Device, a virus filtration technology that offers highly productive parvovirus clearance for monoclonal antibodies and therapeutic proteins. As a result of selective layering, the Viresolve® Pro Device provides an industry-leading performance consistency superior to other virus filtration devices currently…