Finesse Solutions, Inc., a manufacturer of measurement and control solutions for life sciences process applications, announced the launch of SmartVessel, a single-use 3-liter bioreactor vessel. The SmartVessel features integrated single-use sensors for cell culture applications of mammalian, stem, insect, and plant cells. The SmartVessel also has separate electrochemical sensor ports for fermentation and bio-fuels applications.Ā Ā All wetted materials of the SmartVessel are USP class VI-compliant, animal derived component-free, latex-free, BPA-free, and phthalate-free. This stirred tank bioreactor allows working volumes ranging…
Author Archives: BPI Contributor
Pegasusā¢ Prime: Robust Retention after Pressure Interruptions (Stop and Start)
Virus filtration is a vital part of the viral clearance strategy in bioprocesses. In filtration, size exclusion mechanisms are orthogonal to other inactivation or removal techniques and target the physical dimensions of the virus to achieve a high degree of virus reduction. Any flow or pressure interruption during filtration has been shown to increase the risk of virus passage. This application note reviews the PegasusTM Prime virus removal filters and demonstrates robust, high viral clearance in the presence of prolonged…
Reveal Information That Gives Insights: New Approaches to Sub-Visible Particle Characterization
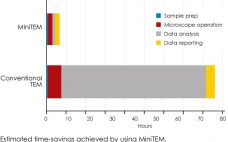
This webcast features: Josefina Nilsson, Head of EM Services,Ā and, Gustaf Kylberg, Product Manager – MiniTEM, Vironova Sub-visible particle characterization is essential when comparing sample quality after different purification steps or for the understanding of product stability. Analysis performed with MiniTEM provides both morphology and accurate quantitative data that can help speed up process and formulation development. In this webinar you will learn: How MiniTEM automatically images, detects and classifies a large number of particles resulting in statistically significant and…
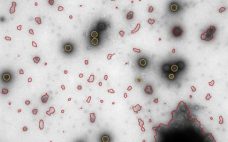
MiniTEM speeds up ātime-to-insightā: automated purity analysis of two different Adenovirus samples
MiniTEMā¢ is a low-voltage transmission electron microscope system designed for nanoparticle characterization. The high-quality images it acquires reveal particle morphologies that can be transformed into accurate metrics. A metric for sample purity based on the ratio of the total area of debris to that of intact Adenovirus particles is presented in this study. The automated analysis that MiniTEM offers encompasses a far larger number of particles compared with studies performed manually using conventional electron microscopy, resulting in accurate and statistically…
A summary of how to develop a cell therapy from concept to market authorization
Cell therapies offer new opportunities for the treatment of disease and injury but considerable infrastructure and a complex range of activities and expertise are required to translate a promising cell therapy product into clinical use. Suppliers, developers, regulators, and care givers need to interact cooperatively in order to bring therapies to patients in the most efficient, safe, and economically viable way and GE Healthcare is working with the Karolinska University Hospital to investigate more effective routes for bringing potential cell…
An Example of Evaluation of protein A Multicolumn Processes for the Capture of Large-Volume, High Concentration Bioreactors, Based on BioSCĀ® Predict Optimization Software
The present paper provides some recent data regarding the optimization of BioSCĀ® processes using three commercially available protein A resins, based on the BioSCĀ® Predict software. A rapid description of the first steps to develop a multi-column process guided by simulation is provided to avoid generating unrealistic conclusions. Other approaches could however be applied depending on the objectives targeted. Examples of multicolumn systems based on three commercially available protein A resins are also provided, for the purification of a 15,000-L…
Lonza and Forty Seven, Inc., Announce Strategic Manufacturing Agreement for Immuno-Oncology Therapeutic Antibody Pipeline
Lonza, a global leader in biological manufacturing, and Forty Seven, Inc., a clinical-stage immuno-oncology company, announced today a strategic manufacturing agreement for the clinical supply of Forty Sevenās therapeutic antibodies. Under the agreement Lonza will manufacture material at its Slough (UK) facility for Forty Sevenās ongoing and future clinical trials. Forty Seven is committed to the advancement of immuno-oncology through the engagement of new and complementary phagocytic pathways that enhance anti-tumor efficacy and selectivity. Its lead molecule, Hu5F9-G4, is a…
Protected:
There is no excerpt because this is a protected post.
Improving Single-Use Bioreactor Design and Process Development: New Research Towards Intensifying Seed-Train and Scale-Up Methods Using 5:1 Turn-Down
This webcast features: Surendra Balekai, Senior Global Product Manager, Thermo Fisher Scientific Bioproduction Operating bioreactor vessels at low working volumes (low turn-down ratio) is often desirable but brings about challenges in regard to mixing, mass transfer, and process control. Research done toward optimizing cell culture has provided methods to improve performance and control when operating under these conditions. Implementing bottom heat exchange, making changes to impeller angle and height, taking advantage of the unique Thermo Fisher drilled-hole sparge design, and…
PROVEO Partnership Expands with Addition of Cerbios-Pharma and Oncotec Pharma for the Manufacture of ADCs
CMC Biologics A/S, a global leader in clinical and commercial manufacturing of monoclonal antibodies and other therapeutic proteins, and IDT Biologika GmbH, a privately-held life-science company with a 95 year history of expertise in research, development and manufacture of biologics for human and animal health, announced the addition of Cerbios-Pharma SA (āCerbiosā) and Oncotec Pharma Produktion GmbH (āOncotecā) to PROVEO, their strategic collaboration for providing a complete and efficient solution to the market for the process development and manufacture of…