This webcast features: Juan Lagos and Steve Tottey, Associate Directors of Upstream and Downstream Process Development, WuXi Advanced Therapies Developing innovative advanced therapies is one of our greatest opportunities to dramatically improve patients’ lives. WuXi Advanced Therapies launched a new world-class adenoassociated virus (AAV) vector suspension platform that complements integrated capabilities enabling cell and gene therapies to be developed, manufactured, and released faster and with greater predictability globally. In this webinar, we will discuss our recent technical advancement on efficient…
Author Archives: BPI Contributor
Challenges Of Host Cell Protein Analysis With ELISA And How To Obtain Better Coverage Of Your ELISA Antibody Reagents
Why is host cell protein (HCP) detection and removal so critical when manufacturing biologics? Product purity and, most importantly, patient safety depends on it. Sandwich ELISA has been a favored method for HCP testing due to its high sensitivity and high throughput, but challenges and limitations exist. Coverage depends strongly on the use of antibody reagents that adequately detect and capture HCPs. In this webinar, we address effective techniques for both optimizing HCP ELISA coverage through the selection of reagents…
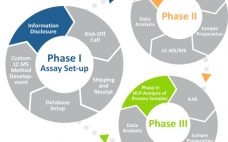
Identification of Host Cell Protein (HCP) Impurities using Antibody Affinity Extraction and Mass Spectrometry Approach
This webcast features: Eric Bishop, Vice President of Research & Development, Cygnus Technologies A well-developed, broadly reactive, and qualified HCP ELISA remains a gold standard method effectively used during the purification process to ensure removal of HCP and to demonstrate process consistency and final drug substance purity. Unfortunately, the ELISA does not inform which HCPs are present or to which HCPs the assay reacts. Mass Spectrometry (MS) is a powerful tool for identifying those HCPs that persist through downstream purification…
Assembly, Labeling, and Packaging Trends: Next Decade’s Sterile Finished Goods Solutions
This webcast features: Thomas Gabriel, Director, Strategy and Business Development, FUJIFILM Diosynth Biotechnologies Learn new trends in packaging, labeling, and distribution of finished goods and supporting technology platforms. At this event, there will be a presentation and dialogue regarding: patient convenience: trends, approaches and demands for future treatments medical device delivery systems of the next decade digitalization’s improvements to patient quality of life and medicinal ease of use manufacturing and supply chain networks of the future. Watch the recorded webcast…
Mixed-Mode Chromatography Resins​ for Biomolecule Purification
This webcast features: Dr. Xuemei He, R&D Chromatography Media Chemistry Manager, Bio-Rad Laboratories Mixed-mode chromatography has emerged as a viable purification method for biomolecules that are otherwise difficult to purify using traditional chromatography platforms and other established means. Scientists are gaining an increased understanding of mixed-mode mechanisms and how these interactions impact selectivity. The industry is becoming more adept at including mixed-mode steps in production processes while also working to address robustness, platform fit, and productivity. This webinar will examine…
Effectively Securing Cell and Gene Therapies with Closed Systems
With their innovative new treatments, cell and gene therapies (CGT) are growing rapidly. But their traditional production processes don’t allow the supply of these therapies to keep up with demand. Historically, these therapies are produced for small patient populations in clinical trials using laboratory scale equipment and utilizing manual, open processes completed under laminar hoods. But manufacturers looking for more efficiency and flexibility are turning to new solutions. One key area of interest is the implementation of a closed system…
Characterization Platform for Structural and Functional Feature Investigation of Bispecific Molecules
Bispecific antibodies (BsAbs ) bind to two or more targets which could be different antigens or different epitopes. Structurally, one type of bispecific antibody is similar to an IgG which contains an Fc region, and other bispecifics do not have an Fc region and typically have a relatively small molecular weight. Therefore, the characterization for a bispecific is more challenging than for a typical monoclonal antibody because of this variation in structure. The poster here discusses a set of platform…
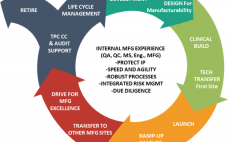
Robust Stable Line Platform for Biologics Development
This webcast features: Dr. Sean Liour, Vice President for Project Management, GenScript ProBio A cell line platform can provide the best solution for the biopharmaceutical target. This webinar explains how to develop a robust stable line platform for CMC projects. GenScript’s biologics platform can provide one-stop service of CMC for the biopharmaceutical target. Watch the recorded webcast now.
Understanding and Controlling Raw Materials Variation in Cell Culture Media
The FDA requires biopharmaceutical manufacturers to understand and control sources of variation according to the risks they present to process and product. Cell culture media and feeds have been shown to affect cellular performance, product quality, or both. The evolution of media formulas to those that are serum-free and chemically defined poses both a challenge and an opportunity. The challenge is identifying which of the many components have the greatest impact on cells and the molecules they produce. The opportunity…
Cryogenic Temperatures and Cell Viability
This webcast features:Â Dr. Peter Kilbride and Dr. Julie Meneghel, Senior Research Scientist and Cryobiologist, GE Healthcare Life Science In this webinar, we will address fundamentals of cryobiology to (1) understand why a cryopreservation protocol is needed, (2) see what happens biologically to cells while cooling, and (3) allow attendees to design an optimized protocol for their cell type and cryopreservation configuration (e.g., sample size). We will cover the importance of applying controlled-rate cooling and how to best tune it to…