This webcast features: Van Leang, Senior Director of Global CMC Operations, HJB Bio Vendors supporting biologics manufacturing are developing equipment to enable continuous process in downstream operations. Using continuous downstream operations reduces operational time and raw material costs. HJB has developed a downstream hybrid process that uses an automated batch approach to achieve a continuous downstream capture process. This allows the use of the current monoclonal antibody (MAb) capture platform but reduces cost overall by adopting the benefits of continuous…
Author Archives: BPI Contributor
Technology-Ready Processes for Gene Therapy Manufacturing 2.0
This webcast features: René Gantier, PhD, Director of Technology, Gene Therapy, Repligen The current manufacturing processes for viral vectors for gene therapy, which we can define as Gene Therapy Manufacturing 1.0 (e.g., adherent cell culture and transient expression from plasmid transfection), are not productive enough to meet the future demand considering the quickly increasing number of approved gene therapies and clinical trials. A transition is therefore ongoing to implement more productive and scalable processes, leading to Gene Therapy Manufacturing 2.0 using…
Why, Why, Why… ELISA? A Look at the Benchmark HCP Assay
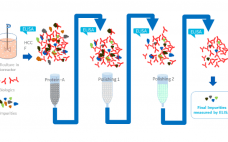
Host cell proteins (HCPs) are a primary source of impurity in biologics manufacturing. When present in drug formulations, HCPs can reduce efficacy, introduce toxicity, and increase risk of long-term immunogenicity. Understanding HCP profiles and integrating effective removal strategies are critical when developing a new biological drug, both for ensuring patient safety and fulfilling regulatory guidelines. HCP populations can be complex and structurally diverse, and most changes in upstream culture conditions affect HCP concentrations and control strategies. Accurate and reliable HCP…
Ask the Expert: Key Considerations for Cryogenic Preservation and T-Cell Viability
Cryopreservation provides critical protection for cell therapies by minimizing genetic changes. But cooling too slowly or quickly risks diminishing cell viability upon thaw. On 11 March 2020, Peter Kilbride (senior research scientist) and Julie Meneghel (cryobiologist), both of Cytiva (formerly GE Healthcare Life Sciences), discussed the importance of controlled-rate cryopreservation. Illustrating how mammalian cells change when frozen, Kilbride and Meneghel offered concrete cryopreservation strategies and identified temperatures at which it is safe to stop controlled cooling and transfer drug product…
Ask the Expert: A Robust, Stable Platform for Biologics Development
Sean Liour (vice president for project management at GenScript ProBio) delivered an Ask the Expert presentation on 18 March 2020 to explore the advantages of his company’s platform for cell-line development. Liour explained that successful biologic development hinges on robust host cell lines, capable expression vectors, and discriminating clone-screening systems. Overviewing relevant technologies and capabilities, Liour illustrated how his company’s ProCLD platform helps sponsors navigate cell-line development. Liour’s Presentation Researchers must weigh their options carefully when selecting a host cell…
Cyclic Cell Harvest with CONTIBAC® SU Filters
As the Biotech industry is moving towards single-use components, larger batch volumes and higher cell concentrations, conventional cell harvest technologies reach their limit. Depth filters, for instance, can only cope with the increasing demands by stacking more filter elements and therefore increasing the footprint and their economic burden. Hence, innovative solutions are needed to keep pushing the boundaries of the biologics production. The CONTIBAC® SU filter of DrM excels where existing technologies crumble. Unlike in any competing technology, the filtration…
Innovative Closed Process CAR-T Cell Therapy Platform to Streamline Approach for Manufacturing with Great Predictability
This webcast features: Tatiana Golovina, Senior Director, Cell Therapy Process Development, WuXi Advanced Technologies For many years, the primary forms of cancer treatment have been chemotherapy, radiation, and surgery. An amazing breakthrough known as chimeric antigen receptor (CAR) T-cell therapy is being studied in the treatment of various types of cancer, including acute and chronic lymphoblastic leukemia, non-Hodgkin lymphoma, myeloma, and solid tumors. Developing innovative advanced therapies is one of our greatest opportunities to dramatically improve patients’ lives. WuXi Advanced…
Assessing Viral Clearance in Early Phase Process Development
This webcast features: William H. Rushton, Process Chromatography Support Scientist, Bio-Rad Laboratories Viral clearance studies are part of a multifaceted approach to ensure the safety of biopharmaceutical products. In order to prevent costly changes to a manufacturing process, it is important to assess each operation unit for its efficiency on the removal or inactivation of adventitious agents early on during downstream process development. A design of experiments (DOE) approach was utilized in this case study to investigate the effect of…
Accelerating Vaccine Development By Innovative Purification Solutions and State of the Art Quality Testing
This webcast features: Sirat Sikka and Florian Durst, Field Application Scientists (Purification and Pharma Analytics), Thermo Fisher Scientific The emergence of new diseases and infections has entailed the need for rapid and efficient development of safe and efficacious vaccines. Additionally, no effective vaccines currently exist for long-known pandemic diseases such as HIV or malaria. To address the challenges the vaccine industry faces, new vaccine modalities such as viral vectors, recombinant protein subunits, and nucleic acids are being researched and developed,…
Want to keep up with demand for CGT testing? Stay nimble and invest, says Eurofins
With record numbers of cell and gene therapies in development, Eurofins BioPharma Product Testing is investing in specialized technologies and scientific talent to tackle technical and regulatory questions around biosafety, characterization, and functionality. The advanced therapy services space is booming, riding the wave of cell and gene therapies (CGTs) entering and progressing through the clinic. While only a handful of such products have reached commercialization, the US Food and Drug Administration (FDA) anticipates numerous approvals in the coming years based…