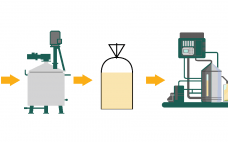
The pipeline for antibody–drug conjugates (ADCs) includes hundreds of candidates, with several expected to receive approval in the near future. As more commercial products reach the market, there is an acute need for CDMOs that understand how to execute late-stage studies to support a filing strategy. CDMOs with plans to support commercial-scale ADC manufacturing are setting up processes to handle challenging supply chains and investing in facilities and processes to ensure efficiency, quality and security. In this white paper, we…
Author Archives: BPI Contributor
Process Intensification: Ways to Achieve Extreme Biologics Volumetric Productivity
This webcast features: Xiao Pan, Director of Process R&D, GenScript ProBio The trend of biologics titer increase brings significant cost reduction. Process intensification needs to be introduced and applied to improve the titer performance and lower the COGS. Besides fed batch, GenScript ProBio has developed process intensification solutions including high-density inoculation and intensified perfusion. Through this webinar, you will learn the following information: The scope and trend of process intensification Intensified perfusion development roadmap Case study of process intensification Just…
The Path Toward Successful Innovation in Gene Therapies
This webcast features: Roland W. Herzog, PhD, Professor of Pediatrics, Riley Children’s Foundation Professor of Immunology, and Director of the Gene and Cell Therapy Program, Indiana University School of Medicine, and Nagendra Venkata Chemuturi, PhD, Scientific Director Research, Global DMPK, Takeda Pharmaceutical Company Gene therapies based on adenoassociated virus (AAV) vectors are emerging as a promising treatment strategy for various diseases, including an inherited form of blindness and spinal muscular atrophy. A major obstacle for the wide use of gene…
The Importance of Real-Time CO2 Monitoring in Cell Culture
This webcast features: Oliver Berteau, Pharmaceutical & Biotech Industry Manager, METTLER TOLEDO Process Analytics Inline dissolved CO2 (dCO2) measurement and monitoring during cell culture offers significant benefits for process control impacting productivity and quality attributes. Real-time dCO2 measurement complements routine dissolved CO2 measurements with a blood gas analyzer (BGA) during preclinical and commercial operations. Animal and insect cells are more or less sensitive to the level of dCO2 during cell culture. Toxic below and beyond specific thresholds, dCO2 is also…
The Critical Steps for Protein Therapeutic Potency Assay Development
This webcast features: Jennifer Lawson, PhD, Global Product Manager, Cell Banking and Testing, Sartorius Potency assays are an important part of the drug development process and are required throughout the lifetime of the product. The potency assay needs to correlate with the mechanism of action and provide an indication of stability. With these requirements comes a myriad of challenges in the development process, especially as therapeutics become increasingly complex. Assay development should be stepwise, starting with proof of concept and…
HCP Analysis by ELISA and Orthogonal Methods in Vaccine and Gene Therapy Development
This webcast features: Jared Isaac, PhD, Sr. Scientist, Chromatography and Mass Spectrometry, Cygnus Technologies Next-generation recombinant vaccines and gene therapy products require clinical and commercial manufacturing of protein antigens or viral vectors produced using cell culture technologies. Regulatory guidelines require testing for cell substrate related impurities, media and purification additives, as well as adventitious agents throughout vaccine and gene therapy development to study the candidate’s purity, safety, and efficacy. While low levels of most impurities can be inconsequential, patient safety…
Autologous CAR T Cell Manufacturing Using a Semiautomatic, Closed, Modular Workflow
Cell-based chimeric antigen receptor (CAR) T cell therapies have rapidly advanced in recent years, with a variety of targets in clinical research and several FDA approved products already on the market. There has been tremendous effort to make CAR T cells more effective, safe, and persistent when treating patients. On the manufacturing side, however, errors, lot-to-lot variation, and contamination can be associated with open processes and manual handling of CAR T cells. Cell isolation, gene editing, expansion, and cryopreservation are…
Edata Exchange – Embark On Your Digital Transformation Journey
You need the right data at the right time from your supplier to make important production and supply chain decisions to achieve your Biopharma Manufacturing goals. Now you have the eMERGE™ Program, our standard platform for the exchange of electronic data. It provides key raw material data, electronically, for every shipment, so you can start making informed decisions right away.
Vero Cell-based Vaccine Production: Cell lines, Media and Bioreactor Options
The recent Covid-19 pandemic introduced new challenges for the vaccine industry, it has also brought in new innovations in vaccine development including DNA/RNA based vaccines. The pandemic also increased the demand for well-established cell-based vaccine production technologies. The article reviews strategies for optimizing Vero cell-based vaccine production using rabies and influenza as examples. The Vero cell line is one of the most satisfactory vaccine production hosts based on its infectability, stability and well-documented performance in quality and quantity of viral…
Simplifying AAV Downstream Process and Product Characterization: A Look at Purification and Analytical Tools
This webcast features: Dr. Julia Baek, PhD, Staff Specialist, Ilaria Scarfone, PhD, MBA, Field Applications Specialist, and Chantelle Gaskin, Field Applications Specialist, Thermo Fisher The optimization of the downstream process for adeno-associated virus (AAV) production with consistent quality depends on the ability to characterize critical quality attributes affecting potency, purity, and safety of the final product. As the gene therapy field continues to push products through the clinical pipeline, an increasing need for efficient analytical tools has become evident. In…