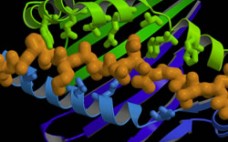
Quality of recombinant/therapeutic proteins produced by engineered cell lines is a concern shared by both manufacturers and regulatory agencies. New assays are needed to facilitate earlier and more rapid analysis of therapeutic protein quality. Cell culture supernatant contains secreted proteins that are indicative of the cellular condition and thus are potential indicators of recombinant protein quality. This study identified changes in secreted host cell proteins (HCP) from cell pools exhibiting varying levels of recombinant antibody expression as determined by quantitative…
Author Archives: BPI Contributor
Global Marketplace
Microbiological Testing Product: GeneDisc specified microorganisms assay Applications: Compendial testing Features: Pall’s GeneDisc specified microorganisms assay is part of its GeneDisc rapid microbiology system based on real-time quantitative polymerase chain reaction (qPCR) technology. Performed using a GeneDisc plate, this rapid assay detects six key indicator microorganisms used in several compendial tests. Users can simultaneously distinguish multiple organisms, obtaining results within two hours (rather than two days with traditional methods) after relevant sample preparation, depending on type of assay performed. Contact…
Biopharmaceutical Development and Production Week
Join us for the week and you will Examine the latest scientific and technical advances to overcome the critical challenges faced by bioprocessing professionals at all stages of development to help you achieve your company’s process and product development goals. Find solutions to the unique challenges of developing and manufacturing therapeutic proteins, that will help you reduce time, cost, and variability during process and product development. Learn approaches to implement the latest analytical technologies that optimize process and product development.…
Global Material Harmonization for Increased Effectiveness and Reduced Risk
As geographic distances continue to metaphorically shrink, there is an increased focus on improving operational effectiveness. But, in order to increase effectiveness, it is important to understand and mitigate risk. In this on-demand webcast, Ben Locwin of Lonza Biologics demonstrates how material harmonization can help reduce risk and increase effectiveness by allowing:
• Use of one material specification
• Shared testing
• Shared raw material qualifications
• And more.
View this webcast to learn more about how synchronization of raw material testing can not only improve effectiveness, but reduce risk and lower costs as well.
Integrity Systems: A Comprehensive Toolkit for Single-Use Solutions
Single-use solutions are in growing demand within the biopharmaceutical manufacturing industry. With a complete line of solutions — from mixing systems to ultra-clean packaging — ATMI LifeSciences is a leader in these technologies. In this educational webcast, Jared Hisle, Global Product Manager at ATMI LifeSciences, explores the full line of Integrity™ Systems single-use technologies, including:
• NewForm™ Sterilizable Packaging
• Integrity™ Liquid and Powder Vessels
• Integrity™ Mixers
• Integrity™ Bioreactors
ATMI maintains the world’s largest installed base of single-use mixing systems and offers the world’s only single-use platform including film extrusion. Join Hisle as he demonstrates the advantages of ATMI’s single-use solutions.
Quality by Design (QbD): Defining Hydrolysates & Designing Quality
Complex. Undefined. Variability. These are all typical attributes that are used to describe protein hydrolysates because they are exactly that — complex, undefined and their performance may vary on a lot-to-lot basis. But despite all of the uncertainties, using hydrolysates as media supplements can stimulate cell growth and improve protein production — and ultimately lower the cost of goods tremendously. That is why the scientists at FrieslandCampina Domo have started the project “Defining Hydrolysates and Designing Quality.”
In this educational webcast, Dr. Jan Boots of FrieslandCampina Domo answers the questions — Can hydrolysates be defined? And can consistent quality be designed into them? Join Dr. Boots as he explores the complexity of hydrolysates and how Domo is working to define them.
Global Marketplace
Automated Osmometry Product: Advanced 20G high-throughput osmometer Applications: Cell culture process development and optimization Features: The Advanced 20G high-throughput osmometer combines state-of-the art osmometry technology and robotics within a parallel sample processing scheme. Samples can be analyzed in a 96-well format within 35 minutes with the same accuracy as stand-alone osmometers. The 20G system supports osmolality testing in even the most demanding cell culture process development schemes in the biopharmaceutical industry. Contact Advanced Instruments Inc. www.aicompanies.com UV Calibration Product: Calibration…
IBC’s Sixth Annual BMD Summit Biopharmaceutical Manufacturing and Development Streamline Facility and Capacity Management, Minimize Downtime, and Mitigate Raw Material Risk
The BMD Summit comprises two in-depth tracks, a combination of programming that will help you increase your manufacturing agility, reduce process variability, and achieve your manufacturing and development goals. Tracks and Session Topics Preconference Workshop: “Mitigating the Risk and Impact of Viral Contaminations: Technical Aspects” (new this year) Track #1, Enabling Efficient Facilities: This track provides you with insights on how to achieve scale and product flexibility. You will hear perspectives from major biopharmaceutical companies on when and where to…
Preclinical Immunogenicity Assessment
Protein therapeutics can potentially elicit immune responses when administered in humans. These antidrug antibodies could result in partial or complete loss of drug efficacy and other complications that have the potential to cause severe adverse effects in the patient. In this webcast, Philippe Stas, Head of Applied Protein Services at Lonza, discusses the benefits of preclinical immunogenicity assessment, including:
• Early-stage Risk Assessment
• Improved Quality and Safety of Drugs
• Reduced Attrition Rate in Drug Development Programs
View this webcast to learn more about managing drug-induced immune responses at the earliest possible stage to produce safer and more cost-effective protein therapeutics.
Geographic Strategies in Biomanufacturing
In BPI’s June issue, we presented a supplement on geographical trends in biomanufacturing. We looked at the influence of a growing demand for biotherapeutics in emerging countries and the influence of new technologies that are driving interest in smaller, perhaps more geographically distributed production. We wanted to explore what a global bioeconomy would look like and where its primary capacity would be concentrated. Authors provided examples of how to balance cost with control issues. They talked about working in different…