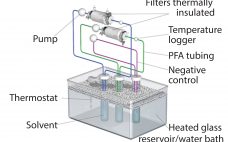
Benefits of single-use technologies over traditional stainless-steel solutions in biopharmaceutical manufacturing include reductions in set-up times, cleaning/cleaning validation costs, elimination of cross-contamination risks, and smaller operating footprints. But despite increasing adoption of such systems, concerns remain about extractable and leachable (E&L) compounds from plastic single-use systems (SUS) components with the potential to compromise the efficacy and safety of final drug products. Such concerns are magnified by the growing number of SUS suppliers and the complex supply chain for SUS and…
Author Archives: James Hathcock
Implementation of the BPOG Extractables Testing Protocols: Working with Multiple Single-Use Components
Single-use technologies offer significant advantages over traditional stainless-steel solutions for biopharmaceutical manufacturing. Reductions in setup times, cleaning and cleaning-validation costs, elimination of cross-contamination risks, and smaller footprints are just some of the benefits they provide. Although adoption of single-use systems (SUS) for commercial manufacturing is expanding, concerns persist that extractable and leachable (E&L) compounds from plastic SUS components potentially can leach into final drug products and compromise efficacy and safety. Those concerns are magnified amid the growing number of SUS…
Regulating Quality in Continuous Processing
Regardless of the industry and product being manufactured, continuous processing has demonstrated numerous benefits. In addition to smaller manufacturing footprints, reduced material consumption and waste generation, increased efficiencies, and lower capital and operating costs continuous manufacturing typically leads to more consistent processes and product quality. In the pharmaceutical industry, the latter two attributes align perfectly with FDA’s Quality by Design (QbD) and process analytical technology (PAT) initiatives. The challenge is determining how to apply these concepts in practice. Applying the…