Over the past 24 months, the leadership of the Bio-Process Systems Alliance (BPSA) has initiated a pilot program to demonstrate the joint feasibility of collecting and sharing pertinent industry business data. We began with “quality-complaint data” centered on the premise that product complaints are tightly associated with product-quality defects in single-use systems (SUSs). This approach can be viewed as “risk assessment 101.” BPSA’s Representation of the Single-Use Industry BPSA is an industry organization primarily comprising drug manufacturers, single-use system suppliers,…
Author Archives: Jeffrey Carter
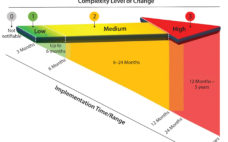
An Industry Proposal for Change Notification Practices for Single-Use Biomanufacturing Systems
Current practices for change notification in the biopharmaceutical industry are neither efficient nor conducive to accelerating the adoption of single-use systems (1, 2). Drug manufacturers (end users) often observe that supplier change data packages lack technical content or detail and that the time allowed for change implementation is too short. Occasionally, customers (end users or next tier in supply chain) learn of changes after the fact, possibly even by happenstance. Suppliers, however, can find the potential affect of a change…