Since the introduction of single-use technologies (SUTs) more than 20 years ago, their benefits have been well recognized, and their adoption has been swift. The ability to customize a solution for every operation, every scale, and every product is an attractive proposition when compared with the fixed, stainless-steel facilities SUTs have replaced. But now customization has gone too far. Custom assemblies are being used when there is no need or benefit, and excessive customization is becoming a key challenge of implementing…
Author Archives: Guy Matthews
Reducing the Complexity of Custom Single-Use Assemblies
Single-use technology (SUT) has been adopted on a global scale since its introduction 20 years ago. Its benefits are well-recognized, and it is a key enabling technology in today’s biopharm world. Thousands of single-use products are now on the market and entire processes are being run in single-use systems. Historically, end users have been encouraged to produce ‘customized’ single-use solutions for each individual application. While this can give the user exactly what they want, it can be at the expense…
Single-Use Systems: Balancing the Need Between Customization and Standardization
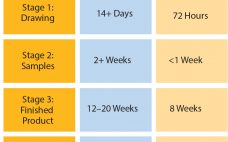
In June 2016, Parker domnick hunter began manufacturing in its new single-use facility in Birtley, Co Durham, UK. Here I discuss the approach taken in the design, testing, and manufacturing of single-use assemblies and some of the advantages they can have in terms of lead times, inventory control, and supply chain robustness. Challenges of Customization For some time, the message relayed to biopharmaceutical manufacturers was to design exactly what you want and need for each process. That meant a customized…