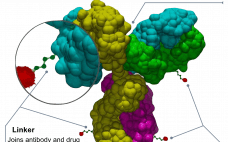
The complex and multiple parts of antibody-drug conjugate (ADC) manufacture means it is integral to choose a CDMO with mAb, small molecule, and bioconjugation expertise, say WuXi Biologics and WuXi STA. The onslaught of ADCs is a hardy perennial in the pages of this publication and others when it comes to predicting the future of medicine. In 2011 when Adcetris (brentuximab vedotin) ā then only the second ADC ā was approved, it was predicted to be the watershed moment and…
Author Archives: Dan Stanton
J&J trial pause does not bode well for viral-vector COVID vaccines, analyst
Johnson & Johnson says the pausing of dosing in trials of its COVID-19 candidate is not unusual, but one analyst believes this could have significant negative impact for viral-vector based vaccines. Johnson & Johnson announced at the beginning of the week it has temporarily paused further dosing in all its COVID-19 vaccine candidate clinical trials due to an unexplained illness in a study participant. This included the Phase III ENSEMBLE trial of JNJ-78436735, also known as Ad26.COV2-S, recombinant, which is…
Lusk for life: Abzena $60m plant to support Ph III and commercial biologics
Abzena has begun operations at its 50,000 square-foot āLuskā facility boasting single-use bioreactors up to 2,000 L to support late-stage and commercial biologics. Contract development and manufacturing organization (CDMO) Abzena has further expanded its biologics offering, opening a $60 million facility in San Diego, California. The site houses a process development laboratory, two cGMP manufacturing cleanrooms for 500 L and 2,000 L scale bioreactors, a GMP warehouse, and analytical development and quality control (QC) laboratories. āWe are already operational and…
Pfizer/BioNTech recruits Rentschler to support COVID-19 vaccine
CDMO Rentschler will be responsible for downstream processing of batches of Pfizer/BioNTechās COVID-19 vaccine candidate BNT162b2 from its site in Laupheim, Germany. BNT162b2 is one of the frontrunners in the race to develop a vaccine against COVID-19. The Phase III candidate is based on messenger RNA (mRNA) and being produced at several facilities in Germany run by developer BioNTech. But now BioNTech and its partner Pfizer have selected contract development and manufacturing organization (CDMO) Rentschler to support production as it…
Navigo and Repligen team on COVID-19 vaccine affinity resin
Protein A-derived affinity ligands against the COVID-19 spike protein coupled to chromatography beads can resolve purification challenges in the development of coronavirus vaccines, say Navigo and Repligen. Bringing a vaccine against COVID-19 to market involves numerous challenges, not least on the manufacturing side. In downstream processing (DSP), the speed of purification and the yield of the purified product are key to ensuring supply of a quality vaccine. As such, bioprocess technology firm Repligen teamed with protein engineering firm Navigo Proteins…
Aji Bio receives FDA approval for commercial oligo process
Ajinomoto Bio-Pharma Servicesā AJIPHASE Technology has received US FDA approval for an undisclosed commercial oligonucleotide, most likely NS Pharmaās Viltepso (viltolarsen). The announcement highlights Ajiās AJIPHASE synthesis technology in its potential to scale up oligonucleotide production. āAJIPHASE is used in the development and cGMP manufacture of large-scale oligonucleotides and peptides,ā a spokesperson from the company told this publication. āThe AJIPHASE technology employs the use of an anchor, instead of a resin, which provides a homogeneous mixture. After the reaction occurs,…
Astrea continues acquisition path picking up purification firm Nanopareil
A transformative year for Astrea Bioseparations has culminated in a second acquisition in a month. Philip Vanek, CTO of parent company Gamma Biosciences, talks to us about the deal, the company, and the changing bioprocess landscape. InĀ November 2019, investment firm KKR announced its intentions to buy Prometic Bioseparations from Liminal BioSciences. In January, the deal closed with renamed Astrea Bioseparations becoming the first company in KKRās $200 million Gamma Biosciences portfolio of life sciences tools subsidiaries. Since then, the firm…
Large-scale SUS āidealā to take on COVID-19 vaccine challenge, says ABEC
ABEC will supply six 4,000 L single-use bioreactors to the Serum Institute of India and says such large-scale systems can produce billions of doses of potential COVID-19 vaccines at reasonable costs. The six Custom Single Run (CSR) bioreactors will be delivered to the Serum Instituteās site in Pune, India and will be used to manufacture Phase II COVID-19 vaccine NVXāCoV2373. The candidate has been developed by Novavax, which last month inked a deal with the Indian vaccine maker to produce…
Guangzhou campus opens doors for bioprocess training
With support from Irelandās NIBRT and bioprocess vendor Cytiva, the Guangzhou Bioprocess Academy has begun training the next generation of biopharma talent in China. In December 2019, this publication reported that a combined $10 million investment from bioprocess vendor Cytiva (then known as GE Healthcare Life Sciences) and Guangzhou Development District government was being used to establish a biomanufacturing training center in the Guangdong province in China. Ten months on and the center, known as the Guangzhou Bioprocess Academy, has…
Novavax and J&J ink COVID-19 vaccine fill/finish deals
To support their COVID-19 vaccine candidates, Novavax has secured space at Endoās facility and Johnson & Johnson has struck a deal with Grand River Aseptic Manufacturing (GRAM). As Novavax progresses its COVID-19 vaccine candidate through the clinic, the firm has inked numerous deals with contract development and manufacturing organizations (CDMOs) to ensure it will be ready for large-scale production. The latest focuses on the fill and finish of the Phase II candidate, NVX-CoV2373, with a deal in place with Par…