Demand for large-scale biomanufacturing following an expansion in Denmark has driven a $2 billion investment to add a further 160,000 L of US bioreactor capacity, says Fujifilm. Through an investment of over 200 Billion Yen ($2 billion) from parent firm Fujifilm Corporation, Fujifilm Diosynth Biotechnologies (FDB) aims to build and open a mammalian cell culture facility in the US in 2025. “In the US, this new investment will yield a 17x increase in mammalian cell culture tanks; our current capacity…
Author Archives: Dan Stanton
BMS cites Lonza plant inspection as factor in liso-cel’s ongoing delay
Lonza says observations made by the FDA at its Texas facility should not further hamper the already delayed approval of Bristol-Myers Squibb’s lymphoma CAR-T candidate liso-cel. Bristol-Myers Squibb (BMS) added lisocabtagene maraleucel (liso-cel), an autologous anti-CD19 chimeric antigen receptor (CAR) T‑cell targeting lymphoma, to its pipeline through its $74 billion acquisition of Celgene. Though a Biologics License Application (BLA) was submitted to the US Food and Drug Administration (FDA) in December 2019, COVID-19-related constraints pushed back regulatory inspections necessary for…
Up on the downstream: Sartorius to buy Novasep’s chromatography division
Sartorius Stedim Biotech says it continues to take on the challenges in efficient downstream processing through the proposed addition of Novasep’s chromatography equipment business. The deal, set to go through in the next few months, will see Sartorius bolster its downstream offering within its Stedim Biotech bioprocessing division. Financial terms have not been disclosed for now, and the acquisition is dependent on antitrust approvals. “The unit to acquire comprises resin-based batch and intensified chromatography systems, and primarily focuses on high-pressure,…
Issues at Italian CDMO leads to US approval delay for Novartis’ inclisiran
The US FDA has issued a complete response letter for Leqvio (inclisiran) due to ‘unresolved facility inspection-related conditions’ at a third-party site run by Corden Pharma. In October 2020, this publication reported the US approval of Novartis’ small interfering RNA (siRNA) therapy for the treatment of adults with hypercholesterolemia or mixed dyslipidemia, Leqvio, was dependent on a regulatory inspection at a third-party manufacturer in Italy. While Novartis spoke of regulatory delays of up to five months caused by COVID-19, it…
LucasPye harnessing strategic partners to bring down CDMO costs
Strategic vendor partnership, lean manufacturing, and cloud-based process control systems will help drive down the cost of making biotherapeutics, says Philadelphia-based CDMO LucasPye Bio. Count the number of contract development and manufacturing organizations (CDMOs) in the US capable of making GMP viral vector products and you get seven, according to Tia Lyles-Williams. They are Lonza, Thermo Fisher, Catalent, Emergent, KBI Biopharma, Fujifilm Diosynth Biotechnologies (FDB), and Lyles-Williams’s own company LucasPye Bio. Meanwhile, only the Emergent, KBI, FDB, and LucasPye have…
French biotech sets up exosome plant to deliver proteins and vaccines
Ciloa says its exosomes can ‘deliver’ large molecules and viral proteins to specific organs and is setting up a clinical plant in Montpellier, France. The €1 million ($1.2 million) investment will be used to create an exosome production unit at Ciloa’s site in Montpellier. The facility will be a technological demonstrator that will be the prototype for larger projects of production of customized exosomes, capable of producing around 1,000 doses to support early clinical trials, the firm told us. Exosomes…
SK Holdings set to jump into cell and gene CDMO space with Yposkesi buy
Korean investment firm SK Holdings is looking to buy Yposkesi to add a cell and gene therapy business to its growing CDMO portfolio. While the deal is not done and financials have not been revealed, SK Holdings is looking to become the majority shareholder in the majority shareholder of Yposkesi, a France-based contract development and manufacturing organization (CDMO) specializing in advanced therapies. If it goes through, Yposkesi will fall under SK’s CDMO holding company, California-headquartered SK Pharmteco, formed last year…
Logan’s single-use run: Thermo Fisher expands Utah bioprocess container site
Thermo Fisher will strengthen its single-use equipment supply chain by upping capacity of 2D and 3D BioProcess Containers (BPCs) at its facility in Logan, Utah. Thermo Fisher has produced single-use BPCs and fluid transfer assemblies – designed for use in the development and manufacturing of therapeutics and vaccines – at the Logan site for 20 years. This week, the life sciences vendor held a virtual event to celebrate an expansion at the site adding 20,000 square feet of additional clean…
Eli Lilly $880m Prevail buy a ‘good entry point’ into gene therapy
As Eli Lilly agrees to buy Prevail Therapeutics adding a pipeline of neuroscience assets, CEO Dave Ricks hints at further acquisitions in the gene therapy space. The deal announced yesterday will see Eli Lilly pay approximately $880 million upfront for New York-based Prevail Therapeutics, with a further $160 million set to be paid upon the first approval of a product. Prevail, launched in 2017, is developing gene therapies to slow or stop the underlying disease process for patients with neurodegenerative…
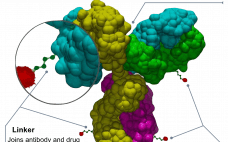
Boehringer Ingelheim re-enters ADC space with $1.4bn NBE buy
Having already dabbled in the antibody-drug conjugate (ADC), Boehringer Ingelheim has gone full in, acquiring NBE-Therapeutics for €1.18 billion ($1.4 billion). The deal sees German biopharma firm Boehringer Ingelheim agree to buy private Swiss biotech NBE-Therapeutics, adding an ADC technology platform and a lead compound NBE-002 in Phase I clinical studies for triple negative breast cancer and other solid tumors. When the deal closes – expected in Q1 2021 – “NBE Therapeutics with its team of highly qualified scientists will…