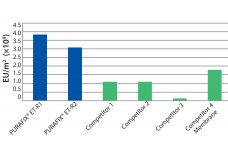
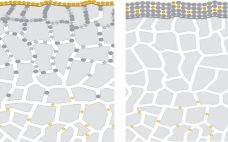
Endotoxins are degradation products from dying gram-negative bacteria and complex aggregates of acidic lipopolysaccharides (LPS). Each is composed of lipophilic lipids and hydrophilic polysaccharides. In humans, endotoxins can cause immune responses such as fever (pyrogenic threshold is approximately 0.1 ng/kg body weight). Unlike bacteria themselves, endotoxins are extremely heat and pH stable and therefore withstand sterilization methods. During protein purification, the reduction of endotoxins is one of the most important and difficult steps. It often includes complex purification strategies (e.g.,…
Author Archives: Corinne Luechinger
Increased Clarification Capacity: Using Filter Aid and FILTRODISCâ„¢ BIO SD for an Economical Filtration
Clarification of fermentation broths is one of the most important steps in bioprocessing. The first purification step after fermentation is the cell harvest, which is designed to remove cells and cell debris as well as to reach maximum product yield in compliance with existing regulatory environments. Standard technologies (centrifugation, separators, membrane, and depth filtration) can no longer handle the high particle loads (>108 cells/mL) in an economical way. Deciding on the right purification system involves addressing questions about process performance,…