The preceding articles have shown how biopharmaceutical companies increasingly are adopting single-use manufacturing technologies for commercial production facilities as their confidence grows in the material science, supply chains, and robustness of single-use systems. Single-use suppliers can support adoption of end-to-end process platforms in commercial manufacturing settings with dedicated teams of experts in process development, engineering, and regulatory support. Advances in process analytical technology (PAT) are providing engineers with greater information on conditions within their single-use bioprocess platforms to allow for…
Author Archives: Chris McCready
Model Predictive Control for Bioprocess Forecasting and Optimization
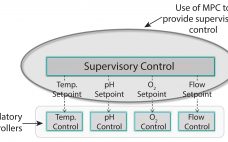
Automation hierarchy in bioprocess manufacturing consists of a regulatory layer, process analytics technology (PAT), and (potentially) a top-level model-predictive or supervisory layer. The regulatory layer is responsible for keeping typical process measurements such as temperature, pressure, flows, and pH on target. In some cases, spectral instrumentation in combination with multivariate analysis (MVA) can be configured to measure parameters such as glucose concentration. A cascade control structure can be set up when the nutrient flow setpoint is adjusted to maintain the…