Continuous and semicontinuous manufacturing systems have been used for many years in numerous sectors — including the automotive, food and beverage, oil refining, chemicals, pulp and paper, electronics, metal smelting, steel making, and waste-water treatment industries. Most of these industries are capital intensive and switched to flow manufacturing to increase productivity and flexibility; reduce cycle times, inventory, waste, and costs; and achieve enhanced product quality. Despite the capital intensive nature of drug manufacturing, the biopharmaceutical industry has lagged behind these…
Author Archives: Cynthia Challener
Preparing for Continuous Bioprocessing: An Interview with Pall Corporation’s Chief Technology Officer Martin Smith
Martin Smith, PhD, has been with Pall for about nine years and assumed the role of chief technology officer at Pall about 18 months ago. He spoke with Cynthia Challener, PhD, about Pall’s biopharmaceutical business unit and how the company is positioning its technology suite for a continuous process paradigm. Smith: There is no doubt in our minds that we see movement toward continuous bioprocessing. When you look across an array of different industries, the move to continuous or parallel…
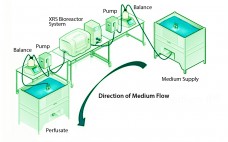
A Look At Perfusion: The Upstream Continuous Process
Although implementation of continuous manufacturing for biopharmaceuticals is in the early stages, continuous cell culture technology has been around for close to thirty years. Perfusion was initially developed in the late 1980s as a means for increasing protein titers (1). However, high costs driven by media consumption limited widespread commercial adoption. In the same time frame, advances in cell line engineering, media composition, and bioreactor design led to 10-fold increases in titers for batch and fed-batch modes, eliminating the first…