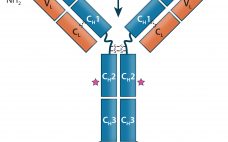
Monoclonal antibodies (MAbs) are bivalent and monospecific, with two antigen-binding arms that both recognize the same epitope. Bispecific and multispecific antibodies, collectively referred to herein as bispecific antibodies (bsAbs), can have two or more antigen-binding sites, which are capable of recognizing and binding two or more unique epitopes. Based on their structure, bsAbs can be divided into two broad subgroups: IgG-like bsAbs and non–IgG-like bsAbs. We have chosen to focus on the former in this review. Part one provides a…
Author Archives: Bryan Monroe
Therapeutic IgG-Like Bispecific Antibodies: Modular Versatility and Manufacturing Challenges, Part 1
Antibody-based immunotherapy has advanced significantly since 1986, when the US Food and Drug Administration (FDA) approved the first mouse monoclonal antibody (MAb) for clinical use: Orthoclone OKT-3 (muromonab-CD3). In the intervening years, researchers have applied the tools of genetic engineering to clone immunoglobulin G (IgG) genes into a number of expression vectors. In the 1990s, the bioprocess industry was able to produce fully human antibodies in cultured cells. As of June 2017, the FDA and the European Medicines Agency (EMA)…