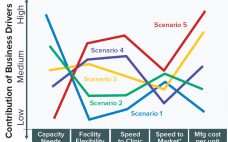
Drug Substance Scenarios Given the complexity of the biopharmaceutical industry and the increasing diversity of products and companies, it is clear that there will be no “one size fits all” solution to biomanufacturing. Instead, we see a range of biomanufacturing scenarios playing out over the next 10 years. Five high-level scenarios were selected for drug substance manufacturing and two for drug product to cover the full spectrum of process and facility types. Each facility type is associated with a representative…
Author Archives: Beth Junker
Minimizing the Environmental Footprint of Bioprocesses
Part 1 of this two-part article introduced the need to reduce the environmental footprint of bioprocesses and evaluated the impact of solid-waste disposal. Part 2 continues by describing the effects of the remaining elements of the bioprocess footprint: wastewater, electricity, and air emissions. Wastewater Process Waste Streams: Generally, raw materials to produce and purify biopharmaceuticals fall into one of the following categories: inorganic/organic salts, sugars/polyols, trace elements, vitamins, amino acids, surface active agents, or complex (undefined)…
Minimizing the Environmental Footprint of Bioprocesses
Biomanufacturers must take active measures to minimize their environmental footprints and promote environmental sustainability. The collateral benefit of reducing environmental footprint often is viewed as only a secondary consideration after cost of goods and product quality. Biopharmaceutical processes are 80% defined by the time of proof-of-concept studies (clinical trial stage 2b). This milestone is before the official technical transfer to commercialization or manufacturing organizations and almost always before the environmental evaluation of a production process. This step is…