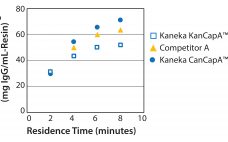
Recent improvements in monoclonal antibody (MAb) upstream process technologies have led to increased product titers (from 5 to 10 g/L) and a corresponding change in impurity levels. To yield highly pure MAb drugs from such high-titer feedstocks, new, robust, and cost-effective purification processes need to be developed to follow this upward trend. KANEKA has developed KANEKA KanCapAâ„¢ 3G, an innovative protein A resin that demonstrates higher binding capacities and allows milder elution conditions than well-known high-binding capacity agarose based resin.…
Author Archives: Koji Iritani
Kaneka Protein A Cellulose
Protein A chromatography is still the preferred capture step in monoclonal antibody (MAb) production because of its high selectivity and robustness, although cation-exchange (CEX) or multimode chromatography techniques are claimed as alternatives. Kaneka Protein A Cellulose increases the value of protein A affinity chromatography for MAb production. Kaneka’s unique combination of a proprietary designed alkaline stable protein A ligand and a highly cross-linked cellulose base matrix meets customer requirements for improved performance, high binding capacity, alkaline and…