With the increase of T-cell immunotherapy research, cell therapy developers have encountered the difficulty of producing consistent, high-quality products. Approved therapies Kymriah™ (tisagenlecleucel, Novartis), Yescarta™ (axicabtagene ciloleucel, Kite Pharma), and late-phase clinical products BB2121 and UCART19 have been critiqued for presenting potential obstacles to patient access because of high costs of treatment and variable manufacturing outcomes. Manufacturing processes need to be developed to increase control over each unit step in a way that improves product consistency over the life of…
Author Archives: Alex Klarer
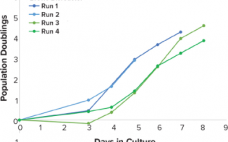
Demonstrating Scalable T-Cell Expansion in Stirred-Tank Bioreactors
Emerging cell therapies have excited the pharmaceutical industry because they indicate potential new pathways to treat some of the most life-threatening diseases. T-cell therapies currently are the flagship technology in cell therapy with recent US FDA approvals of Novartis’ Kymriah (tisagenlecleucel) and Gilead’s Yescarta (axicabtagene ciloleucel) treatments. Those therapies and others still in development use peripheral blood isolated lymphocytes (PBLs) modified with chimeric antigen receptors (CARs) or modified T-cell receptors (TCRs) to trigger the innate cytotoxic response of these immune…